Controlled release bioactive agent delivery device
a bioactive agent and controlled release technology, applied in the field of delivery devices, can solve the problem that the device itself does not provide any other significant function, and achieve the effect of reducing the risks and disadvantages of more invasive surgical techniques, and minimizing damage and interference with body tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Release of Triamcinolone Acetonide from Stainless Steel Wires
[0167] Three different polymer solutions were prepared in tetrahydrofuran (THF) in the manner provided below in order to provide coating compositions in the form of a one-part system. The three solutions contained varying amounts of poly(ethylene-co-vinyl acetate), with a vinyl acetate content of 33% (w / w), relative to the amount of poly(n-butyl)methacrylate, with an approximate weight average molecular weight of 337 kD. Each of the three solutions contained a constant amount of triamcinolone acetonide relative to the total polymer weight.
[0168] The coating compositions were prepared as follows. The polymers were initially added to the THF and dissolved overnight while mixing on a shaker at 200 revolutions per minute (rpm) at room temperature (approximately 20° C. to 22° C.). After dissolution of the polymer, the triamcinolone acetonide was added, and the mixture was placed back on the shaker at 100 rpm for 1 hour, to fo...
example 2
In Vitro Release of Triamcinolone Acetonide from Helical Coils
[0177] Two different solutions were prepared in tetrahydrofuran (THF) as in Example 1. The compositions prepared are summarized in Table III:
TABLE IIICoating Compositions applied to the Helical Coil.Parts by weightWeight of coatedCoatingComposition(pbw)composition (μg)Coating 1dTA / pEVA / pBMA50 / 27.5 / 22.51950Coating 1eTA / pEVA / pBMA50 / 40 / 101928
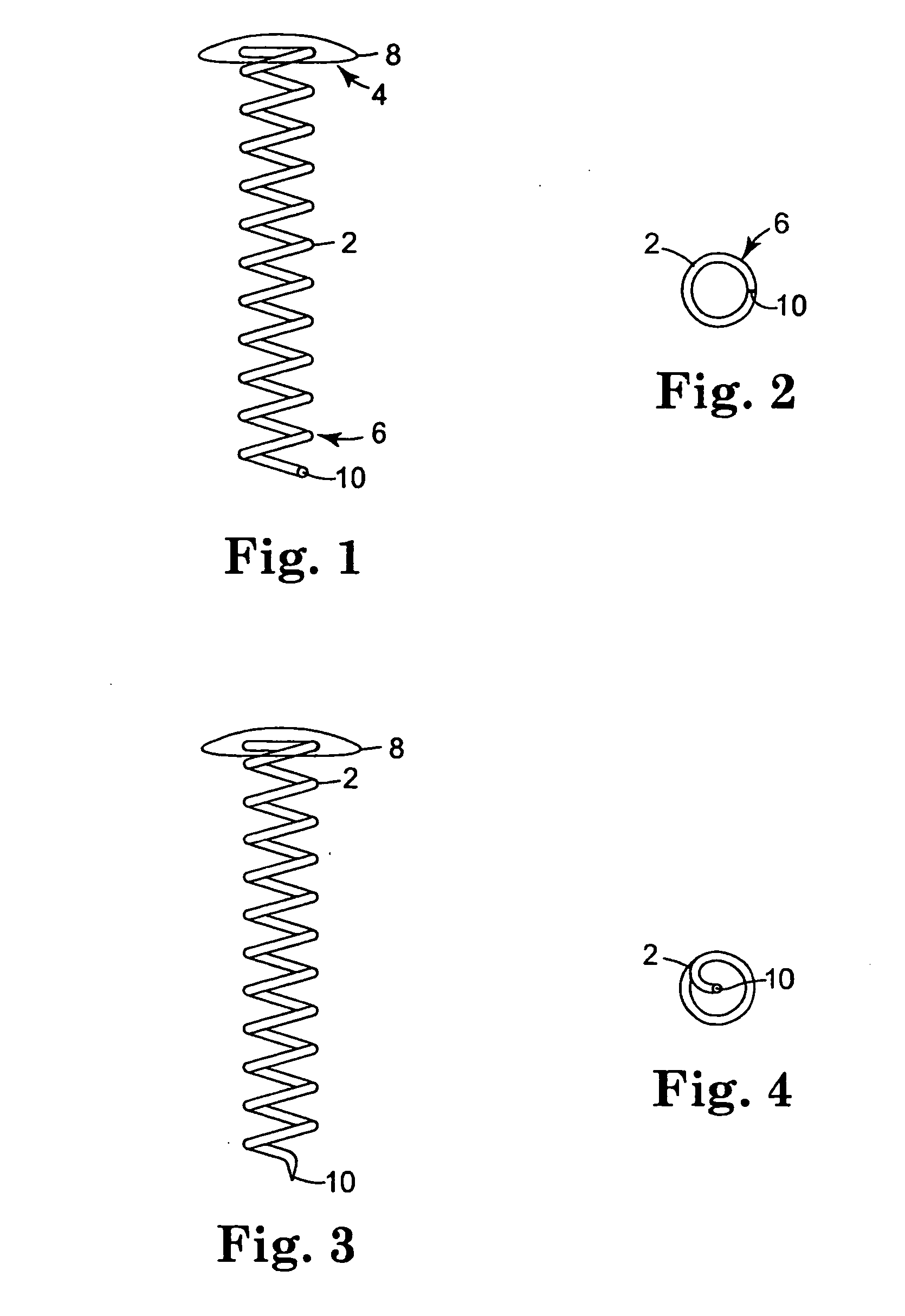
[0178] Helical coils with attached caps were fabricated from the alloy MP35N™ (commercially available from ESPI, Ashland, Oreg.). The coils were cleaned in an alkaline solution, then rinsed with deionized water. The coils underwent additional cleaning using an isopropyl alcohol wash and rinse. The coils were dried and weighed prior to coating.
[0179] Solutions for Coatings 1d and 1e were sprayed onto the coils using ultrasonic coater equipment that consisted of an ultrasonic spray head (Sono-Tek Milton, N.Y.) and syringe pump system for the coating solution. A pin vise was used to hol...
example 3
In Vivo Release of Triamcinolone Acetonide from Helical Coils
[0184] Ten coils were coated with two different formulations, Dose A and Dose B, and were implanted into the vitreous chamber of rabbit eyes to provide sustained release of triamcinolone acetonide. Table V summarizes the coating compositions applied to the coils in this Example. Dose B was designated a “fast release” coating, and this coating composition included a relatively larger ratio of pEVA to PBMA, as compared to the “slow release” Dose A coating composition.
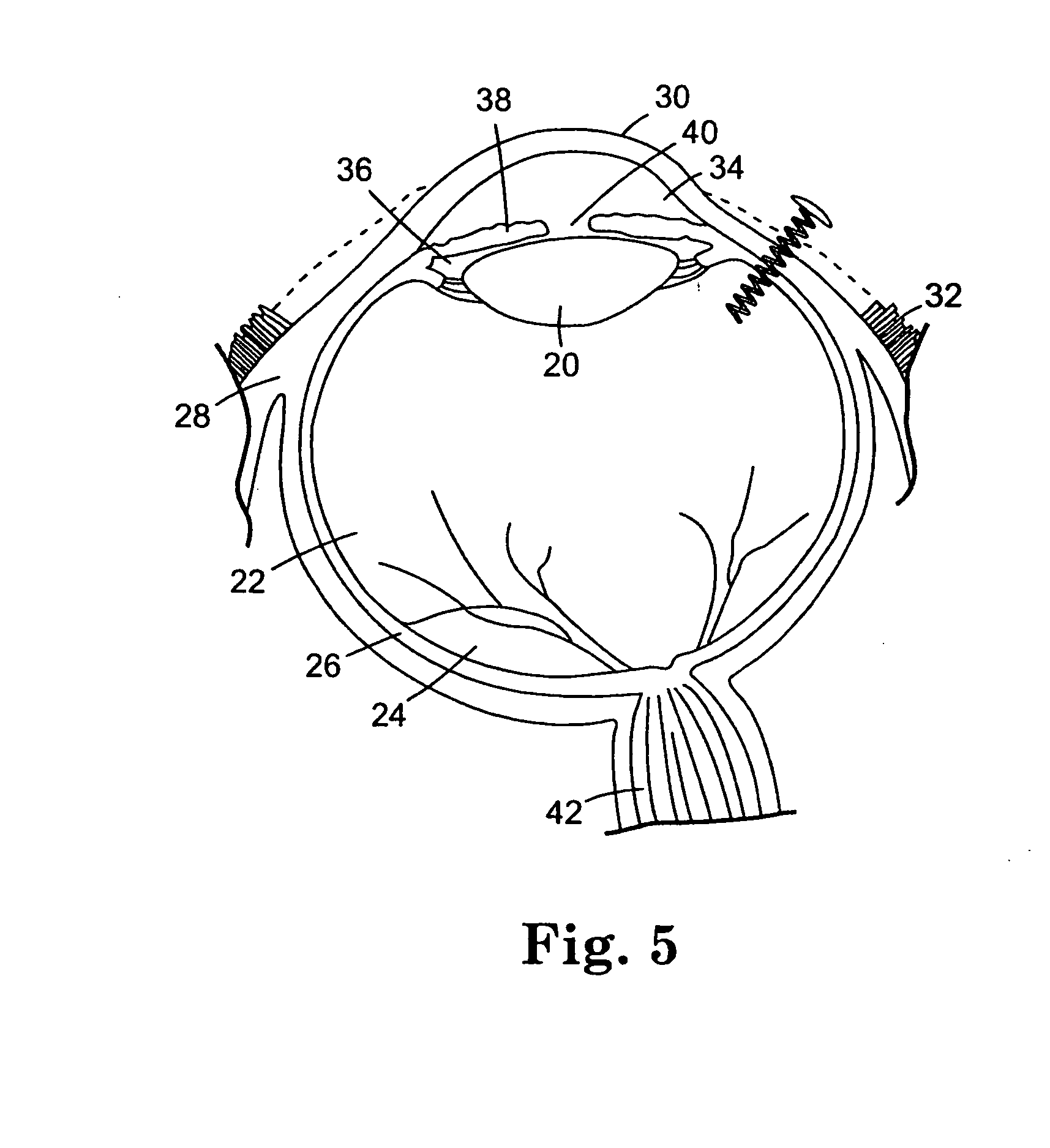
[0185] The coating solutions were prepared according to the procedure described in Example I. The coating solutions were applied to the coils according to the procedure described in Example II. The coated coils were implanted into the vitreous chamber of rabbit eyes as follows. The conjunctiva was dissected and pulled away from the incision site, and an incision was made into the eyes utilizing a needle stick through the sclera. A self-starting coil that inclu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com