Polymeric compositions and related methods of use
a polymer composition and composition technology, applied in the field of moussel adhesives, can solve the problems of complex synthetic procedures, no surface modification strategy that can be universally applied, and current methods rely on expensive instruments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Succinimidyl Carbonate PAO, SC-PAO7
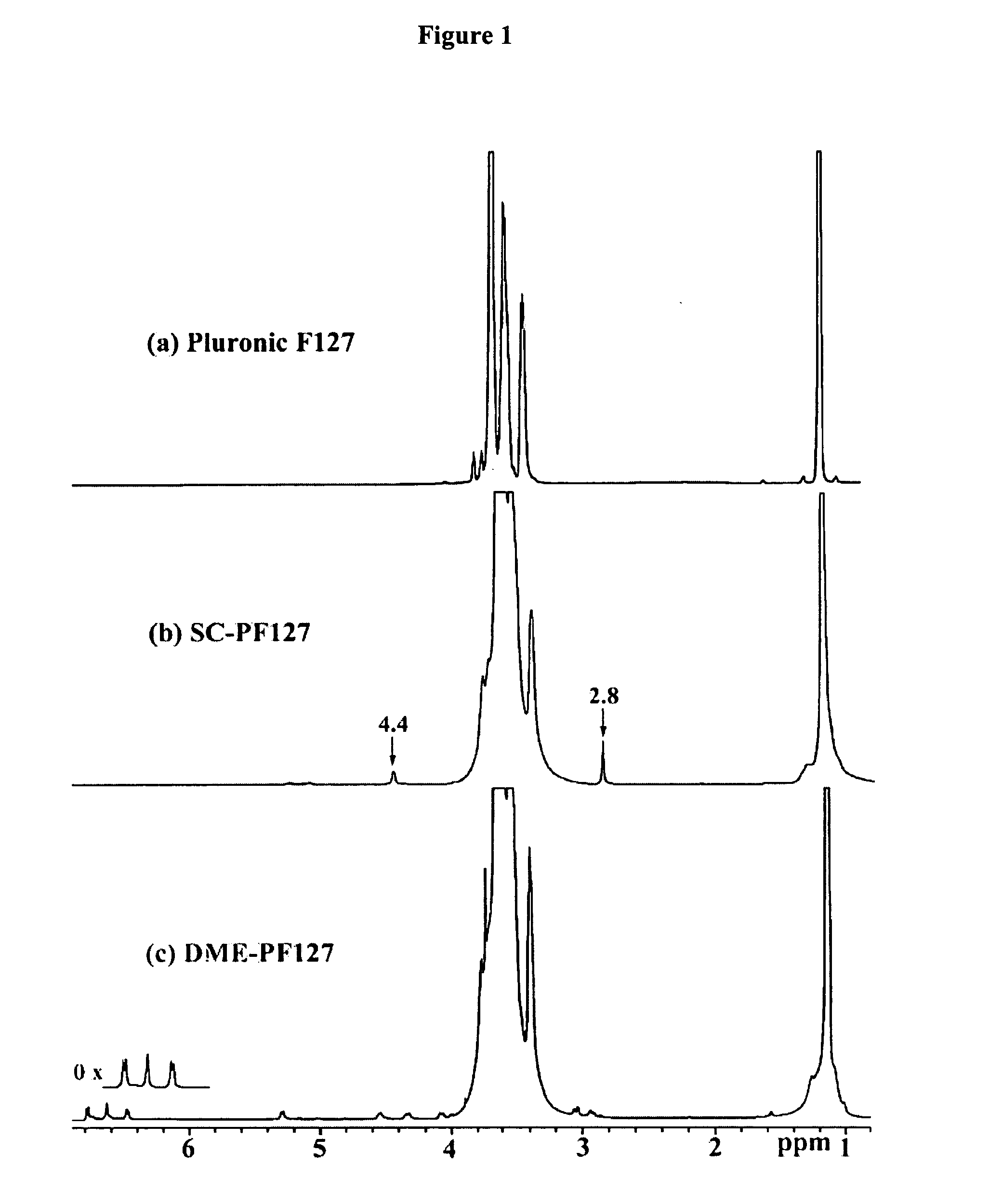
[0168] PLURONIC® F 127 (0.60 mmols) was dissolved in 30 mL of dry dioxane. N,N′-Disuccinimidyl carbonate (6.0 mmols) in 10 mL dry acetone was added. DMAP (6.0 mmols) was dissolved in 10 mL dry acetone and added slowly under magnetic stirring. Activation proceeded 6 hours at room temperature, after which SC-PAO7 was precipitated into ether. The disappearance of the starting materials during the reaction was followed by TLC in chloroform-methanol (5:1) solvent system. The product was purified by dissolution in acetone and precipitation with ether four times. The product yield was 65%. 1H NMR (500 MHz, CDCl3): δ ppm 0.96-1.68 (br, —OCHCH3CH2O—), 2.80 (s, —COON(CO)2(CH2)2), 3.15-4.01 (br, —OCH2CH2O—; —OCHCH3CH2O—), 4.40 (s, —OCH2CH2OCOON(CO)2CH2CH2—).
example 2
Synthesis of DME-PAO7
[0169] A slurry of DOPA methyl ester hydrochloride (1.25 mmols) and triethylamine (2.5 mmols) was mixed with SC-PAO7 (0.16 mmols) in 10 mL chloroform. The disappearance of the starting materials during the reaction was followed by TLC in chloroform-methanol-acetic acid (5:3:1) solvent system. After stirring for 1 hour at room temperature, the solvent was evaporated off, and DME-PAO7 was purified by precipitation from cold methanol three times. DME-PAO7 gave a positive Arnow test indicating the presence of catechol hydroxyl groups. The product yield was 75%.
[0170]1H NMR (500 MHz, CDCl3): δ ppm 0.98-1.71 (br, —OCHCH3CH2O—), 2.83-3.06 (m, —NHCHCH2C6H3(OH)2COOCH3), 3.15-4.02 (br, —OCH2CH2O—; —NHCH(CH2C6H3(OH)2COOCH3), 4.05-4.35 (d, —OCH2CH2OCONHCHCH2C6H3(OH)2COOCH3), 4.55 (br, —NHCHCH2C6H3(OH)2COOCH3), 5.30 (d, —NHCHCH2C6H3(OH)2COOCH3), 6.45-6.80 (1s, 2d, —NHCHCH2C6H3(OH)2COOCH3).
example 3
Synthesis of DOPA-PAO7
[0171] L-DOPA (1.56 mmols) was added to 30 mL 0.1 M Na2B4O7 (pH=9.32) aqueous solution under Ar atmosphere, followed by stirring at room temperature for 30 minutes. SC-PAO7 (0.156 mmols) in 5 mL acetone was added to the resulting mixture and stirred overnight at room temperature. The solution pH was maintained with sodium carbonate during the reaction. The disappearance of the starting materials during the reaction was followed by TLC in chloroform-methanol-acetic acid (5:3:1) solvent system. The solution was acidified to pH 2 with concentrated hydrochloric acid and then extracted three times with dichloromethane. The combined dichloromethane extracts were dried with anhydrous sodium sulfate and filtered, and dichloromethane was evaporated. The product was further purified by precipitation from cold methanol. DOPA-PAO7 gave a positive Arnow test indicating the presence of catechol hydroxyl groups. The product yield was 52%. 1H NMR (500 MHz, CDCl3): δ ppm 0.92-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com