Novel galectin sequences and compositions and methods utilizing same for treating or diagnosing arthritis and other chronic inflammatory diseases
a technology of galectin and composition, applied in the field of new galectin sequences, can solve the problems of complex discrimination between “harmful” inflammatory cells, inability to identify and treat “harmful” inflammatory cells, and inability to achieve the effect of reducing the inflammatory response in the individual
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
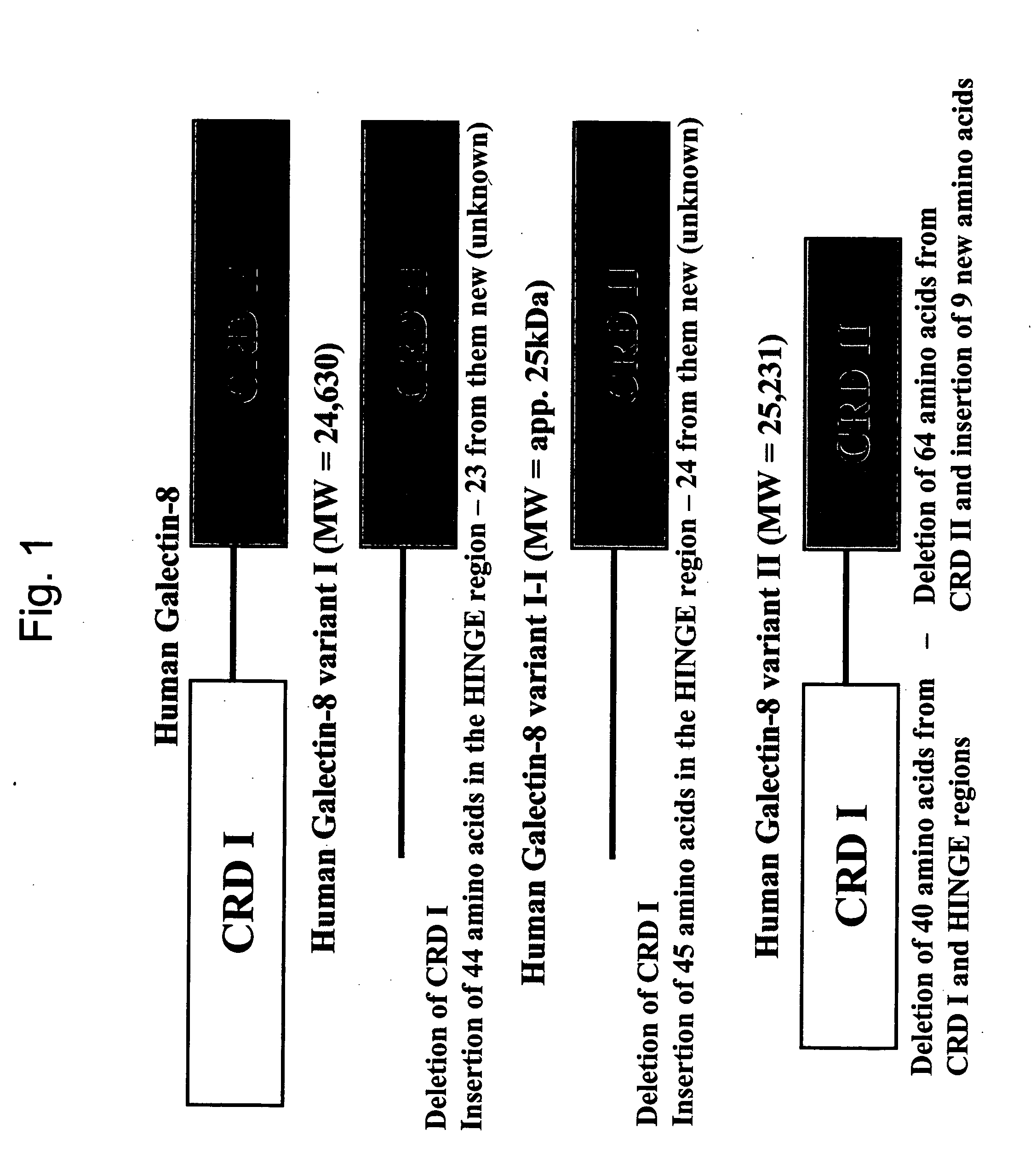
Cloning of Galectin-8 and Galectin-8 Variants
[0269] Materials and Methods
[0270] A. Cloning of Galectin-8
[0271] RNA Preparation: Total RNA of mononuclear cells (MNC) derived from synovial fluids of RA patients was extracted using the Qiagen RNeasy kit (Qiagen, USA).
[0272] RT-PCR: 1 μg of the extracted RNA was reverse transcribed, and PCR-amplified using M-MuLV reverse transcriptase (first strand cDNA synthesis kit, Pharmacia) and Taq DNA polymerase (2.5units) (Gibco-BRL). The reactions were carried out in a DNA thermal cycler 480 (Perkin Elmer Cetus) in a final volume of 50 μl using oligonucleotide primers complementary to a translated region of human galectin-8: [sense primer 5′-AAGAATTCGCCGCCACCATGATGTTGTCCTTAAAC-3′ (SEQ ID NO:1), antisense primer 5′-AATCTAGACTACCAGCTCCTTACTTC-3′ (SEQ ID NO:2)].
[0273] The above described reaction mixture was subjected to an amplification program of 1 min at 94° C., 1 min at 60° C. and 2 min at 72° C. for 30 cycles.
[0274] Purification of PCR P...
example 2
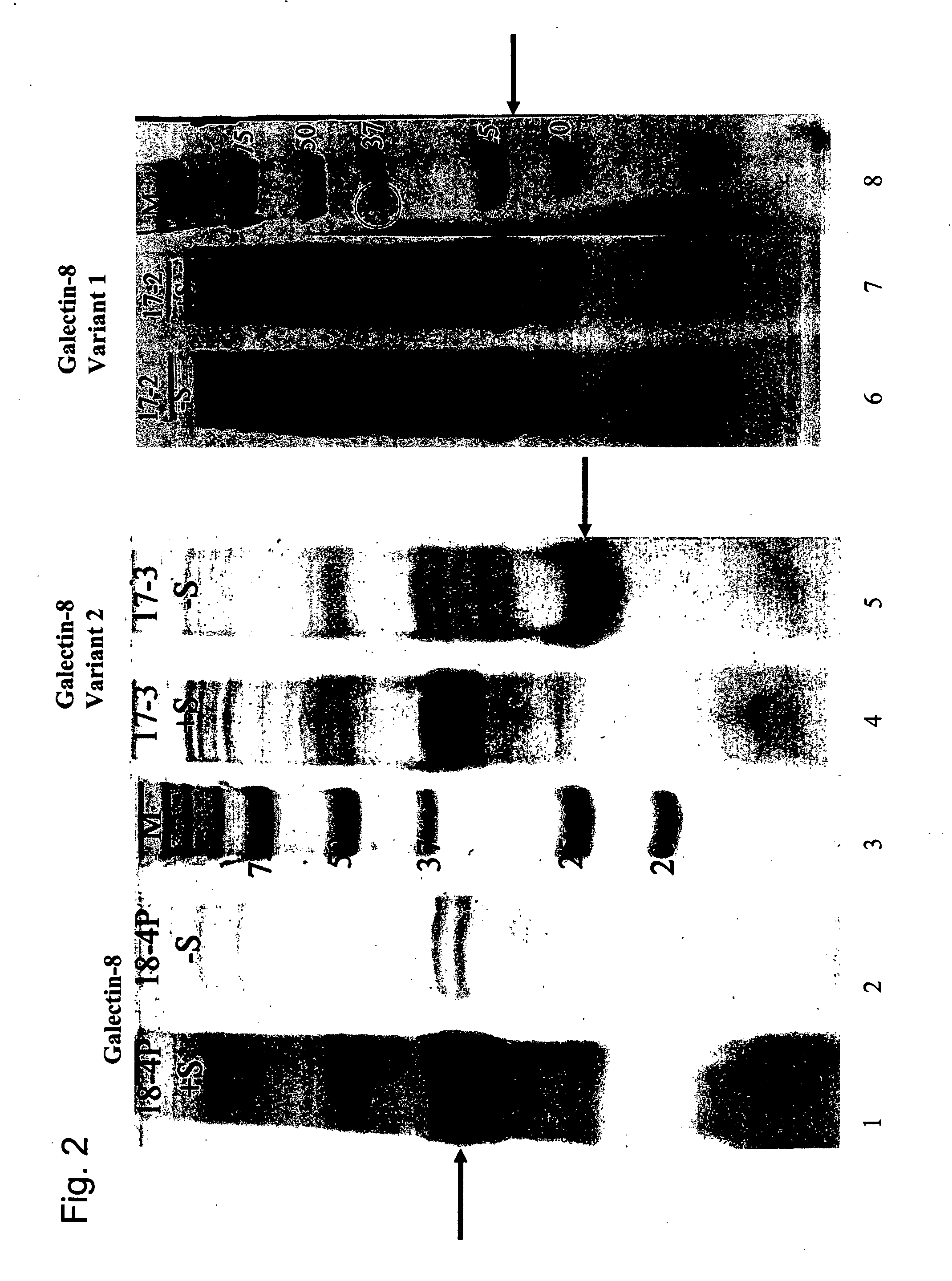
Expressing Recombinant Galectin-8 Molecules in Escherichia Coli
[0283] Materials and Methods
[0284] In order to express recombinant-galectin-8 molecules (r-galectin-8 (SEQ ID NO: 4), r-galectin-8 variant1 (SEQ ID NO: 6), r-galectin-8 variant 2 (SEQ ID NO: 8)) in E. coli, the following primers were used to amplify the entire coding sequence of human galectin-8 using the cloned cDNA in pGEM vector (Promega) as a template: [0285] sense primer: 5′ACTCTAGAGCCGCCACCATGATGTTGTCCTTAAAC-3′ (SEQ ID NO:11), [0286] antisense primer: 5′-CCATATTCCTACCAGCTCCTTACTTC-3′ (SEQ ID NO:12).
[0287] In order to express recombinant-galectin-8 variant 1.1 molecule (SEQ ID NO:16) the following primers were used: [0288] sense primer: 5′CCTCTAGAATGATGTTGTCCTTAAAC-3′(SEQ ID NO: 13); [0289] antisense primer: 5′-GCCATATGCTACCAGCTCCTTACTTC-3′ (SEQ ID NO:14),
[0290] (The Xba I and Nde I restriction sites, respectively, in the above primers are underlined).
[0291] The PCR products were digested by Xba I and Nde I, ge...
example 3
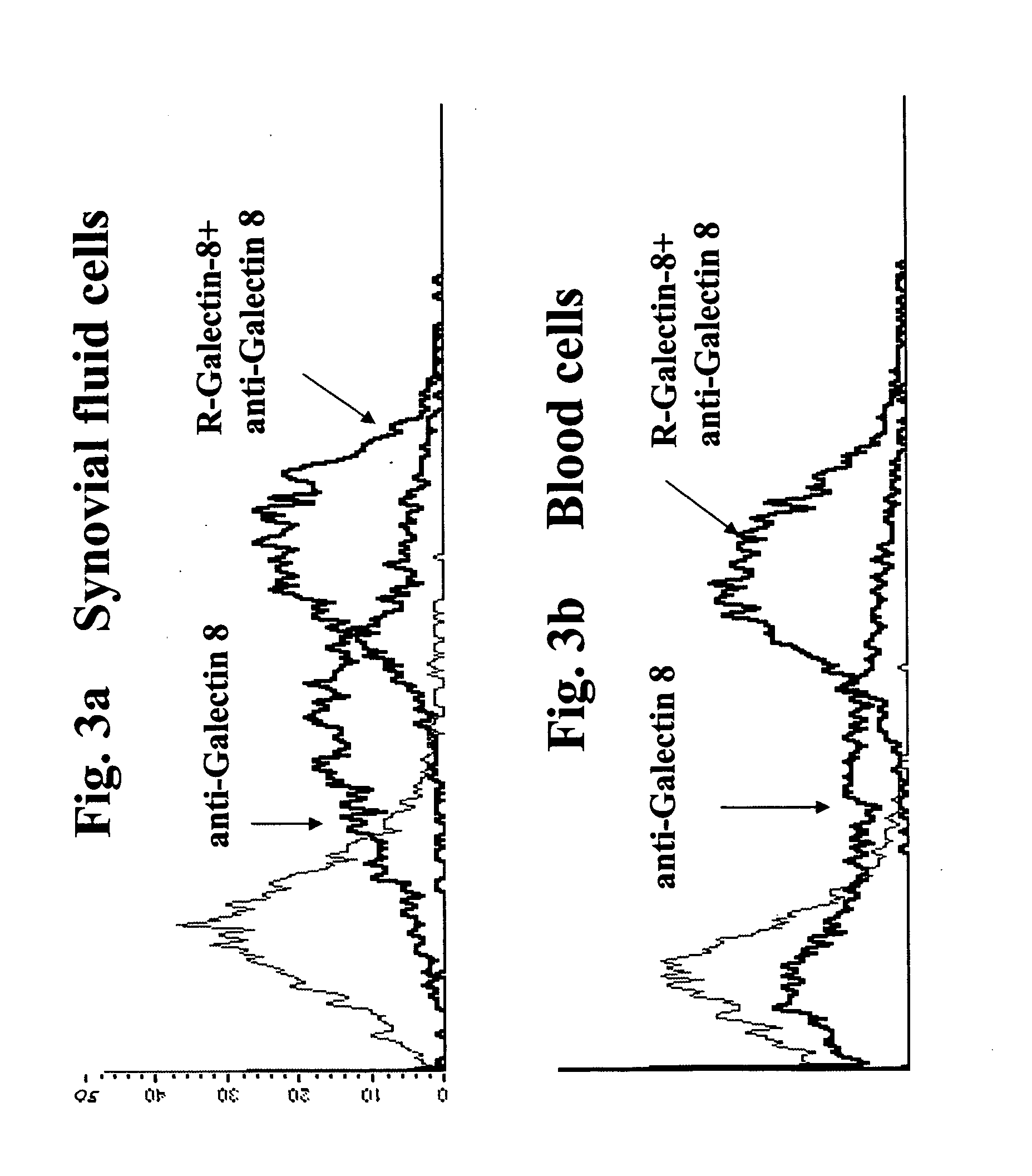
Flow Cytometry Analysis of Galectin-8 Expression on Synovial Fluid Cells and PBLs and Recombinant Galectin-8 Binding to Such Cells
[0295] Materials and Methods
[0296] Peripheral white blood cells and the total cell population from synovial fluids of arthritic patients were isolated (approximately 107 cells) on a Ficoll gradient (Robbins Scientific Corporation, Sunngvale, Calif., USA) and analyzed by flow cytometry using anti-galectin-8 polyclonal antibody (generated in rabbits according to prior art protocols).
[0297] Specific binding of anti-galectin-8 was performed by the incubation of cells for 20 minutes on ice in a binding calcium buffer containing a saturating concentration of anti-galectin-8. Thereafter, a second incubation with a goat-anti-rat conjugated to fluorescein isothiocyanate (FITC) was effected. After incubation, the cells were pelleted, and analyzed in FACS analyzer (Beckton Dickinson, San Jose, Calif.). Following the initial analysis, recombinant galectin-8 (as pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com