Fusion polypeptide suitable as a cytotoxin
a cytotoxin and polypeptide technology, applied in the field of fusion polypeptides, can solve the problems of low detection efficiency, lack of proficient targeting, and high dose requirements, and achieve the effects of improving detection efficiency, reducing the risk of cancer, and improving the survival ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0043] Generation of Fusion Polypeptide Constructs for Testing Putative Oncotoxins for their Effects on Mammalian Cells
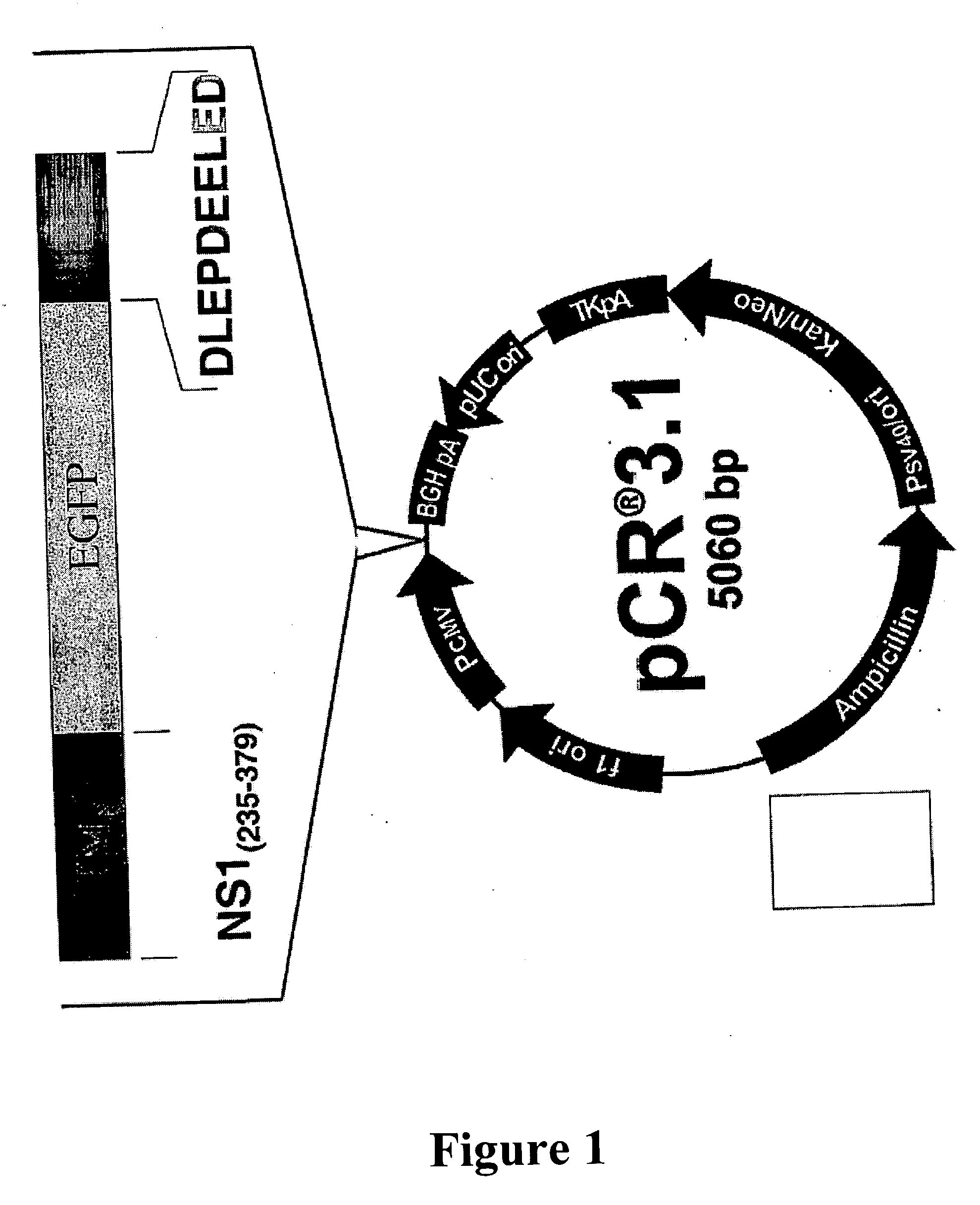
[0044] Given the supposition that NS1 works as a scaffold protein, connecting the catalytic subunit of casein kinase II (CKIIα) to tropomyosin, artificial peptides were designed harboring the tropomyosin binding region of MVM NS1 and connecting either CKIIα (or variants thereof) or just a known CKIIα binding site (FIG. 1). PCR-derived fragments composed of a tropomyosin binding site (TMB) derived from the parvovirus MVMp NS1 protein (amino acids 235 to 279), the stabilizer polypeptide EGFP derived from pEGFP (Becton Dickinson, Heidelberg), and either a casein kinase IIα binding site (derived from CKIIβ: DLEPDEELED) or the functional casein kinase 11 catalytic subunit CKIIα (NCB1 L15618) isolated from the mouse fibroblast cell line A9. (CKIIB) were cloned directly into pCR3.1 (Invitrogen, Karlsruhe), due to 3′adenosinetriphosphate overhangs generated by Taq-polymera...
example 2
[0046] Toxicity Assays
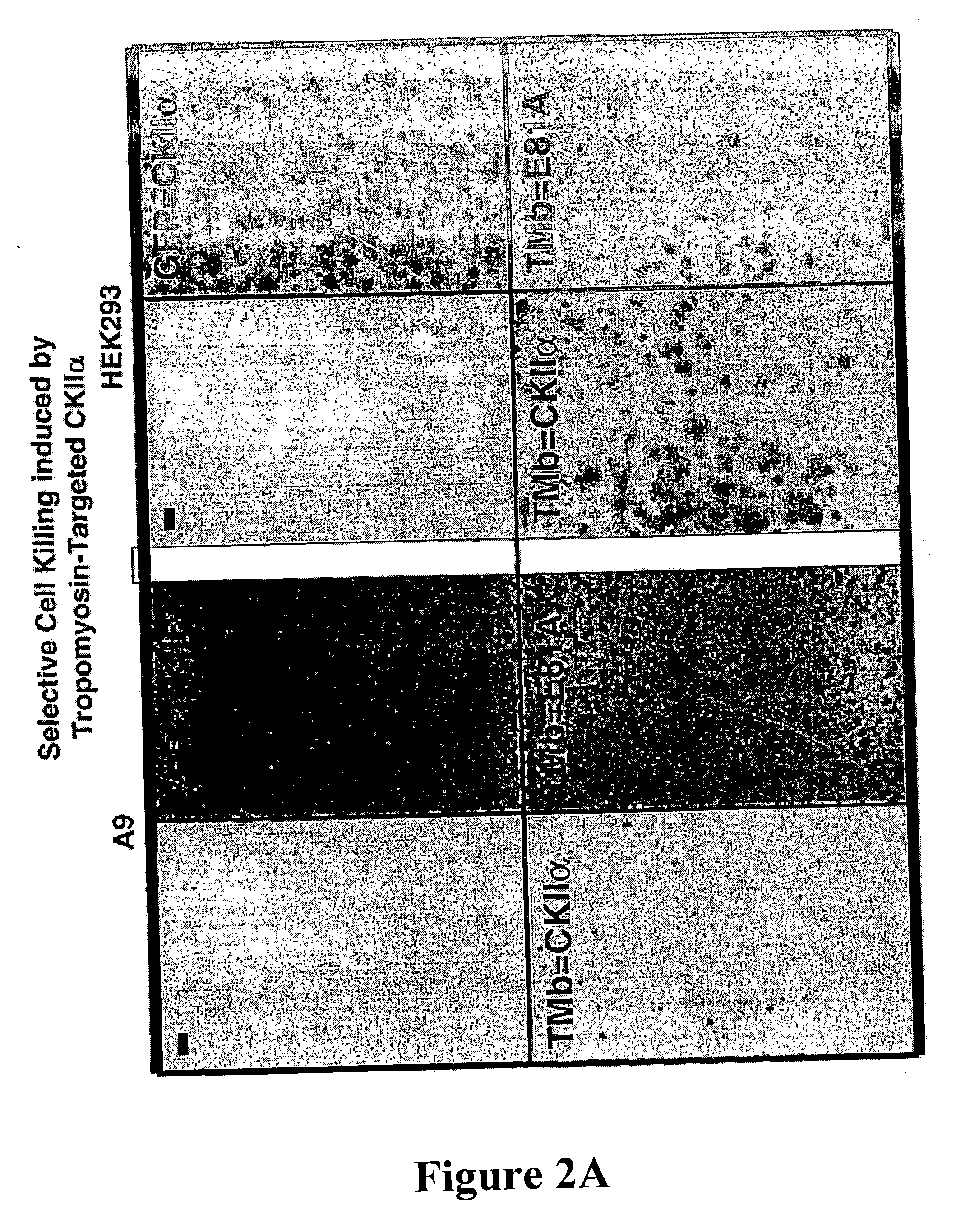
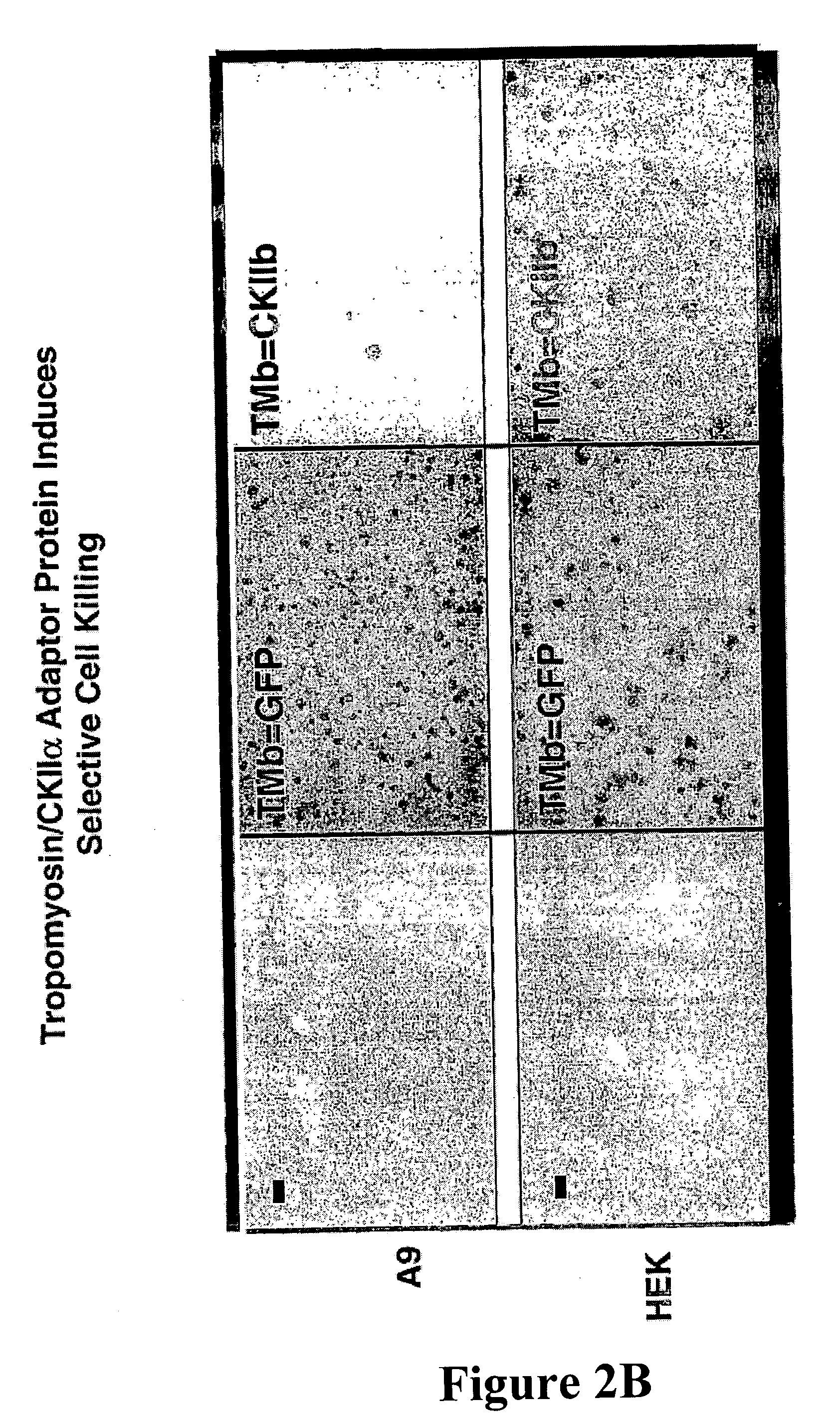
[0047] Colony formation inhibition assays were performed with the constructs described in Example 1. A9 or HEK293 (2×105 cells per 25 cm2) were transfected with 10 μg plasmid DNA using 25 μl lipofectamin in DMEM without serum according to the manufacturer's conditions (Invitrogen). After 5 hr incubation transfection medium was replaced with DMEM containing 10% FBS and cells were grown for additional 48 h in absence of G418 before subdividing into 150 cm2 plates where transfected cells were selected for by addition of 400 μg / ml G418 (SIGMA, Taufkirchen). Growing colonies were fixed and stained according to McCoy after two to three weeks growth under selective pressure. Two representative experiments are shown in FIG. 2a and FIG. 2b. While expression of the two effector proteins (TMB=CKIIα (FIG. 2a) and TMB=CKIIB (FIG. 2b) allowed only few colonies to be generated in A9 cells in comparison to the control peptides, almost similar transfectants were generated in a...
example 3
[0049] Generation of Semi-Synthetic Toxins by Chimeric PCR
[0050] Fusion constructs are generated by consecutive PCR reactions using overlapping primer pairs. In a first reaction the individual PCR-elements generated: TMB(GFP): Lefthand primer A 5′-GATATCCCATGGGGAAAACTAACTTTTTAAAAGAAGGCGA-3′ (SEQ ID NO: 3) with righthand primer B: 5′-TCCTCGCCCTTGCTCACCATATGGCAACTTAACATAGGT-3′ (SEQ ID NO: 4) using pdBMVp (Kestler et al, 1999) as a template. (TMB)-GFP.CKIIB: C: 5′-ACTATGTTAAAGTTTGCCATATGGTGAGCAAGGGCGAGGA-3′ (SEQ ID NO: 5) with D: 5′-GCGGCCGCTCTAGATTAATCTTCCAATTCTTCATCGGGTTCCAAATCCCTCC GATGCTTGTACAGCTCGTCCATGCCGAG-3′ (SEQ ID NO: 6) using pEGFP (Becton Dickinson) as a template. GFP-(CKIIα): E: 5′-CCCGGGATGGTGAGCAAGGGCGAGGAGCTGTTCACCGGGG-3′ (SEQ ID NO: 7) and F: 5′-TCCTCGCCCTTGCTCACCATCTGCTGAGCGCCAGCGGCAGG-3′ (SEQ ID NO: 8) using pEGFP as a template. (TMB)-GFP-(CKIIα): Primer A and F using pEGFP as a template (GFP)-CKIIα(wt or E81A): G: 5′-CTGCCGCTGGCGCTCAGCAGATGGTGAGCAAGGGCGAGGA-3′ (SEQ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
| Nucleic acid sequence | aaaaa | aaaaa |
| Fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com