Cytoplasmic antigens for detection of Candida
a technology of cytoplasm and antigens, applied in the field of method and a means of diagnosing candida infection, can solve the problems of few definitive clinical signs or symptoms, patients that are now surviving major injuries, and significant causes of life-threatening infections in hospital patients, and achieve the effect of simple and rapid method of diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Candida Antigen
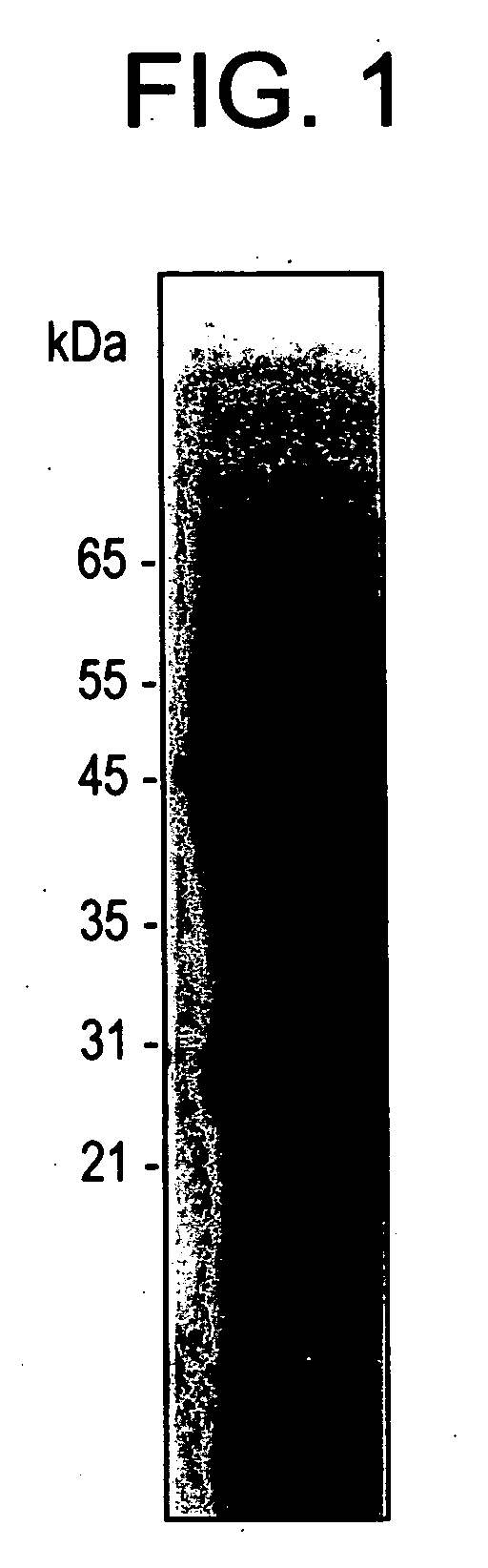
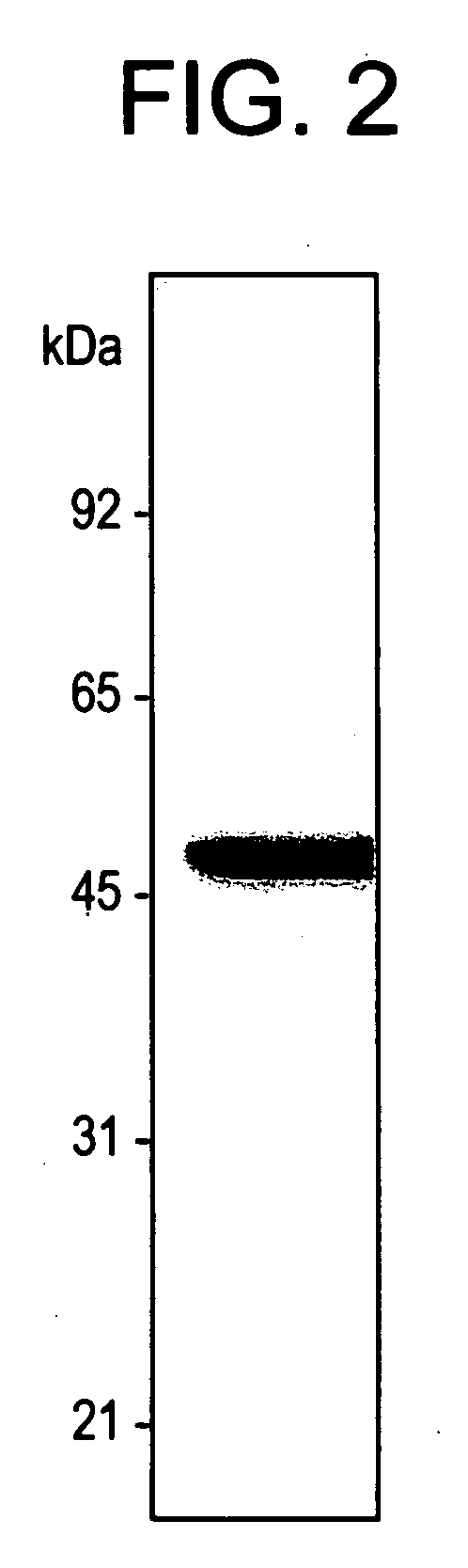
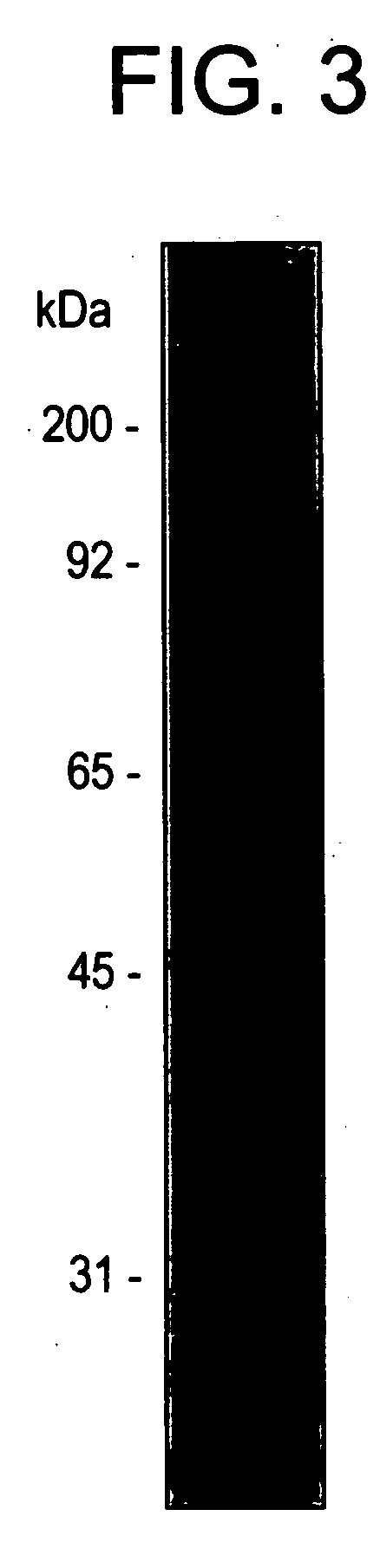
[0092] The following three types of Candida antigen were prepared:
[0093] 1). Cell wall antigen (including mannose);
[0094] 2). Total cytoplasmic antigen (mannose depleted); and
[0095] 3). Purified immunodominant antigen (enolase).
[0096] A clinical isolate of the Candida albicans, was obtained from a patient with vaginal thrush. The identity of the Candida species was confirmed with the use of an API 20C Auxonagram strip (API System S.A., France). The C. albicans isolate was designated KEMH5.
[0097] 200 ml YEPD culture medium (1% yeast extract, 2% peptone, 2% D-glucose) was inoculated with the isolate as a starter culture and incubated for 24 h at 30° C. with aeration. The starter culture was then used to inoculate a 10L YEPD culture incubated under similar conditions in a 23L Bio-Flo Fermenter IV System (New Brunswick Scientific, Edison, N.J.).
[0098] The Candida culture was harvested from the Bio-Flo fermenter system and separated from culture mediu...
example 2
Enzyme Linked Immunosorbent Assays (ELISAS)
[0110] A serum panel was collected from 1998 to 2000 from various patients with Candida infections. Negative control (Control) sera (n=20) were obtained from the Red Cross Blood Bank, Perth, Australia and was obtained from healthy males in the 19 to 25 year age group. Sera (n=13) from patients with recurrent vulvo vaginal candidiasis (VVC) were obtained from King Edward Memorial Hospital, Perth, Australia. Sera (n=108) from patients with oral candidiasis were obtained from Clinipath Ltd and the UWA Dental School, Perth, Australia. Sera (n=39) from patients (n=28) with systemic candidiasis were obtained from Princess Margaret Hospital, Perth, Australia and Prince of Wales Hospital, Sydney, Australia.
[0111] In the case of patients with oral and vaginal Candida infection, confirmation of infection was made by physical examination and by culture of Candida organisms from the relevant body site. In the case of patients with systemic infection,...
example 3
Clinical Evaluation in France
[0124] Clinical evaluation of the triple antigen test kit as described in Examples 1 and 2 was undertaken in the Department of Parasitology and Medical Mycology at the University of Grenoble Faculty of Medicine, Grenoble, France using stored sera.
[0125] Sera from two groups of patients were analysed: those that were blood culture positive and those that were blood culture negative. When possible, sera were taken before, at the time of and after the first day of positive blood culture to be tested. The blood culture negative group was divided into 3 subgroups: Patients that were colonised with Candida and were serology positive, patients that were colonised with Candida and were serology negative, and patients that were not colonised with Candida and serology negative. The sera were obtained from patients hospitalised between 1998 and 2000.
[0126] The triple antigen ELIZA test (“the Applicant antigen test”) was performed according to Example 2. The cut-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weights | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
| molecular weights | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com