Fluoroether compositions and methods for inhibiting their degradation in the presence of a lewis acid
a technology of lewis acid and composition, which is applied in the field of stable, anesthetic fluoroether composition, can solve the problems of high corrosion of skin and mucous membranes, toxic hydrofluoric acid, and the stability of fluoroethers, and achieve the effect of preventing the degradation of fluoroether compounds and effective stabilizing amounts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Activated Alumina as a Lewis Acid
[0032] Type III glass consists mainly of silicon dioxide, calcium oxide, sodium oxide and aluminum oxide. Aluminum oxide is a known Lewis acid. The glass matrix is normally inert to sevoflurane. However, under certain conditions (anhydrous, acidic), the glass surface can be attacked or altered, exposing sevoflurane to active Lewis acid sites such as aluminum oxide.
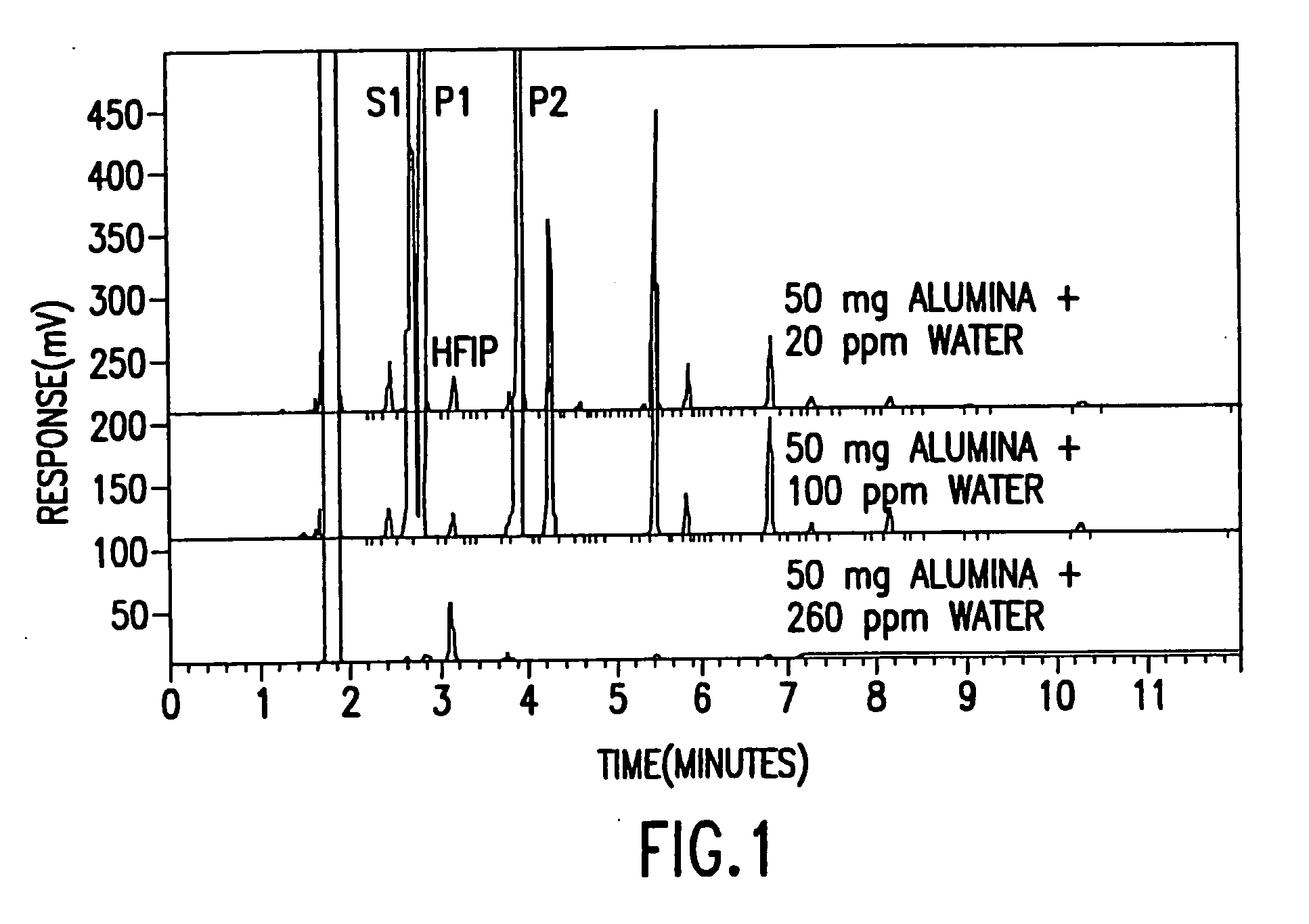
[0033] The effect of water on the degradation of sevoflurane was studied by adding various amounts of activated alumina to 20 ml of sevoflurane containing the following three levels of moisture: 1) 20 ppm water—measured water, no additional water added; 2) 100 ppm—spiked; and 3) 260 ppm water—spiked. Table 1 below shows the experimental matrix.
TABLE 1123A50 mg Al2O3 50 mg Al2O3 50 mg Al2O320 ppm Water100 ppm Water260 ppm WaterB20 mg Al2O3 20 mg Al2O3 20 mg Al2O320 ppm Water100 ppm Water260 ppm WaterC10 mg Al2O3 10 mg Al2O3 10 mg Al2O320 ppm Water100 ppm Water260 ppm Water
[0034] It will ...
example 2
Degradation in Ampules of Sevoflurane by Heat with and Without the Addition of Water
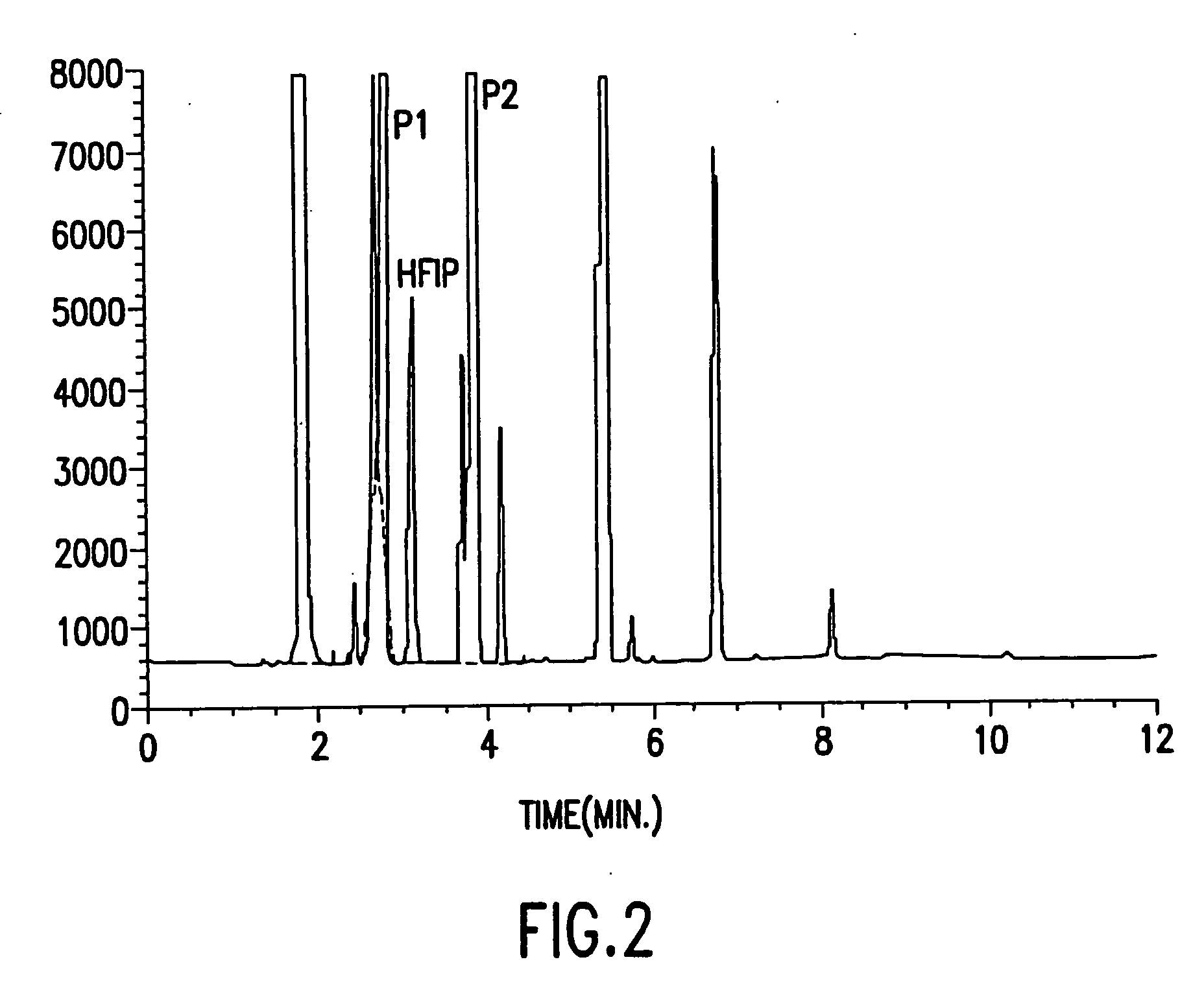
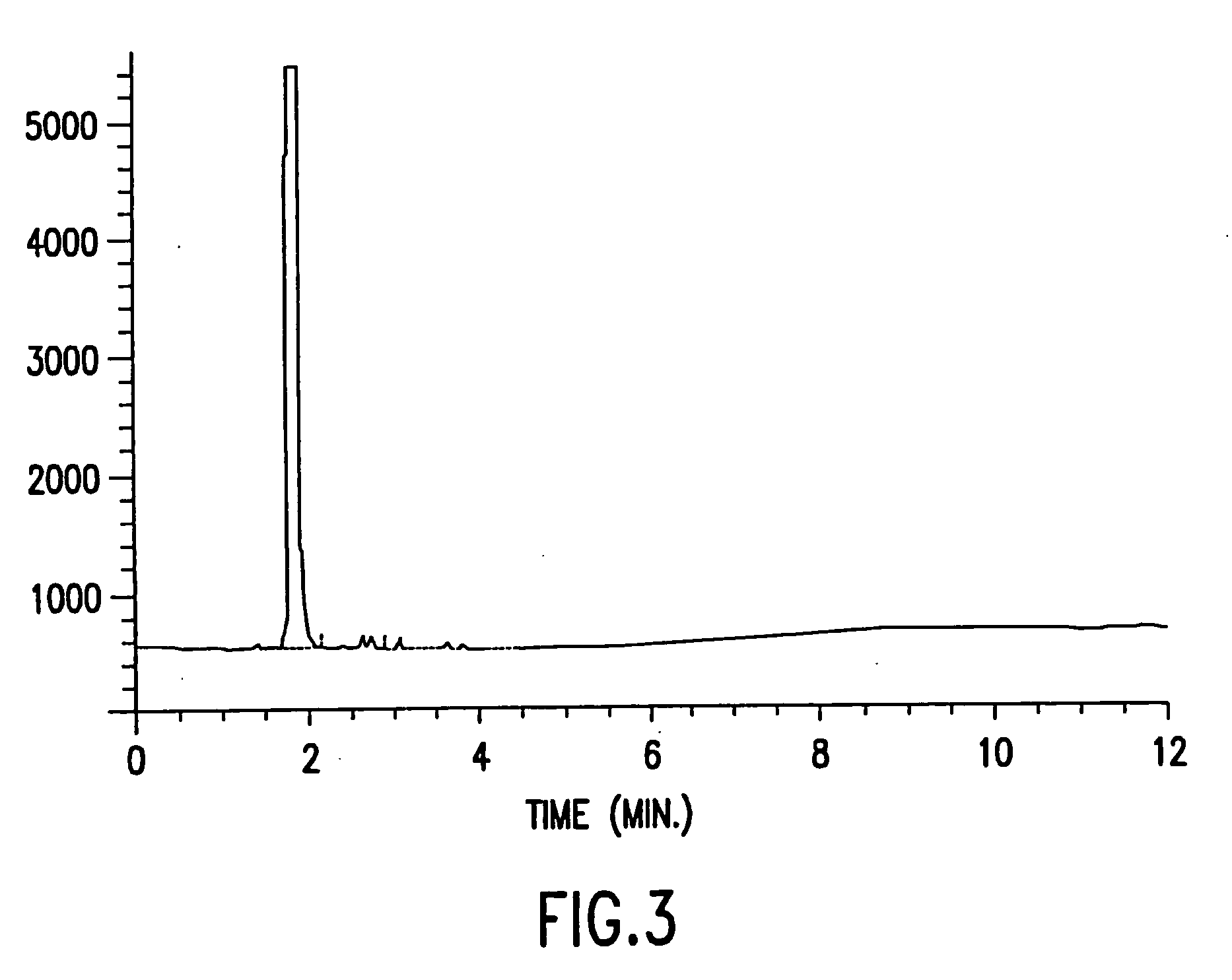
[0035] Approximately 20 mL of sevoflurane was added to a 50 mL Type I clear ampule and approximately 20 mL of sevoflurane and 1300 ppm of water was added to a second ampule. Both ampules were flame-sealed and then autoclaved at 119° C. for three hours. The contents of the two ampules were then analyzed by gas chromatography. FIG. 2 shows that the sevoflurane in the first ampule degraded. FIG. 3 shows that the sevoflurane in the second ampule did not degrade as a result of the Lewis acid inhibitor, namely the added water.
example 3
Degradation of Sevoflurane in Ampules using Water-Spiked Studies (109 ppm to 951 ppm)
[0036] Type I clear glass ampules were used to study the effect of various levels of water in inhibiting the degradation of sevoflurane. Approximately 20 mL of sevoflurane and different levels of water ranging from about 109 ppm to about 951 ppm were added to each ampule. The ampules were then sealed. A total of ten ampules were filled with sevoflurane and varying amounts of water. Five of the ampules were included in Set A and the other five ampules were included in Set B. The anpules were then autoclaved at 119° C. for three hours. Samples in Set A were placed on a mechanical shaker overnight to allow the moisture to coat the glass surface. Samples in Set B were prepared without equilibrating the water with the glass surface. Several control samples were also prepared. Two non-autoclaved ampules (Control Amrpule 1 and Control Ampule 2) and a bottle (Control bottle) were each filled with 20 mL of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com