Method for the crystallization of human serum albumin
a technology crystallization method, which is applied in the field of human serum albumin crystallization method, can solve the problems of inability to perform crystallization of albumin in a sufficiently controlled or reliable way, prevent the development of extensive knowledge of crystallization conditions necessary in the design of large-scale crystallization, and low ionic strength. , the effect of high ionic strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A Preferred Crystallization Process
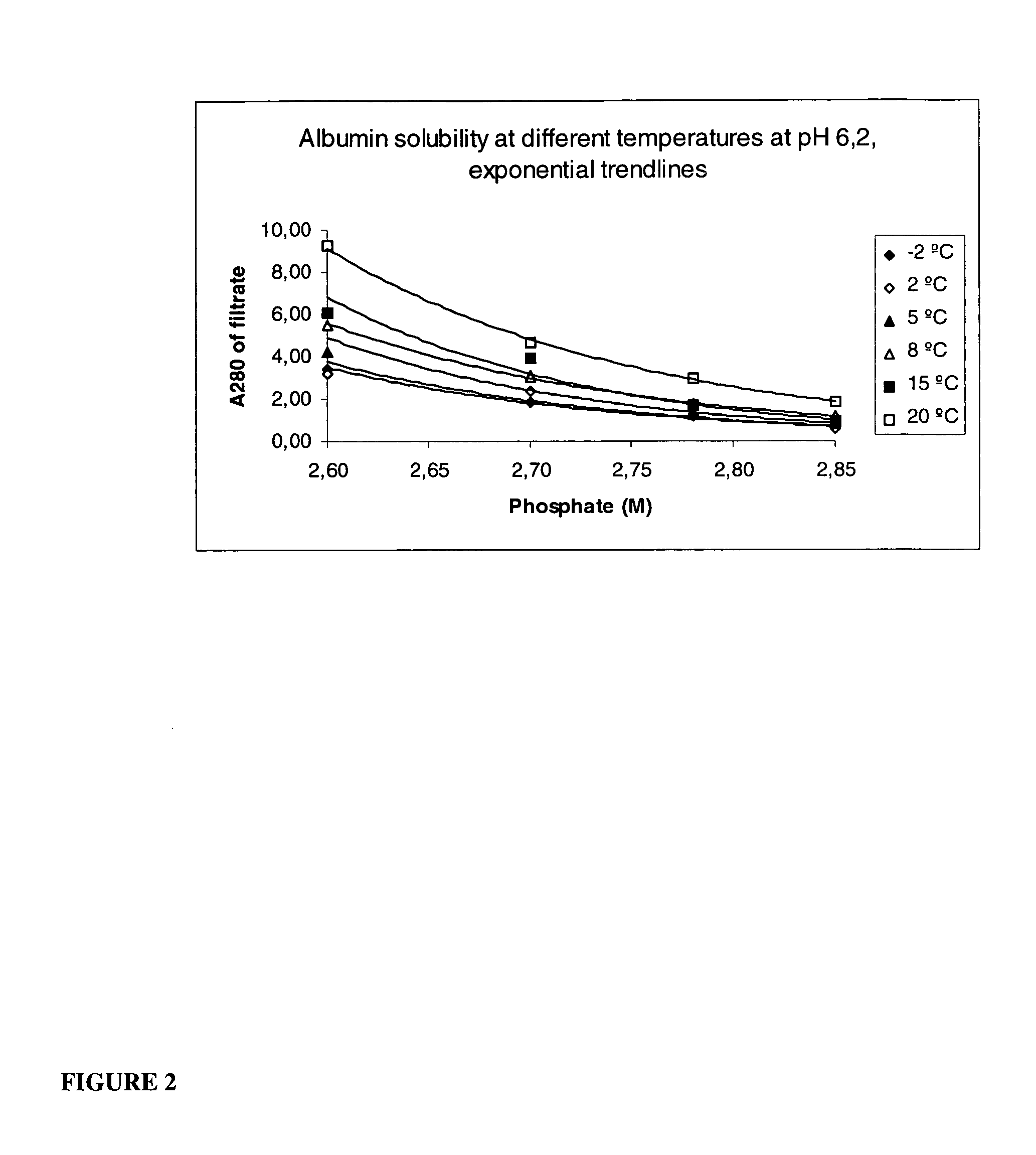
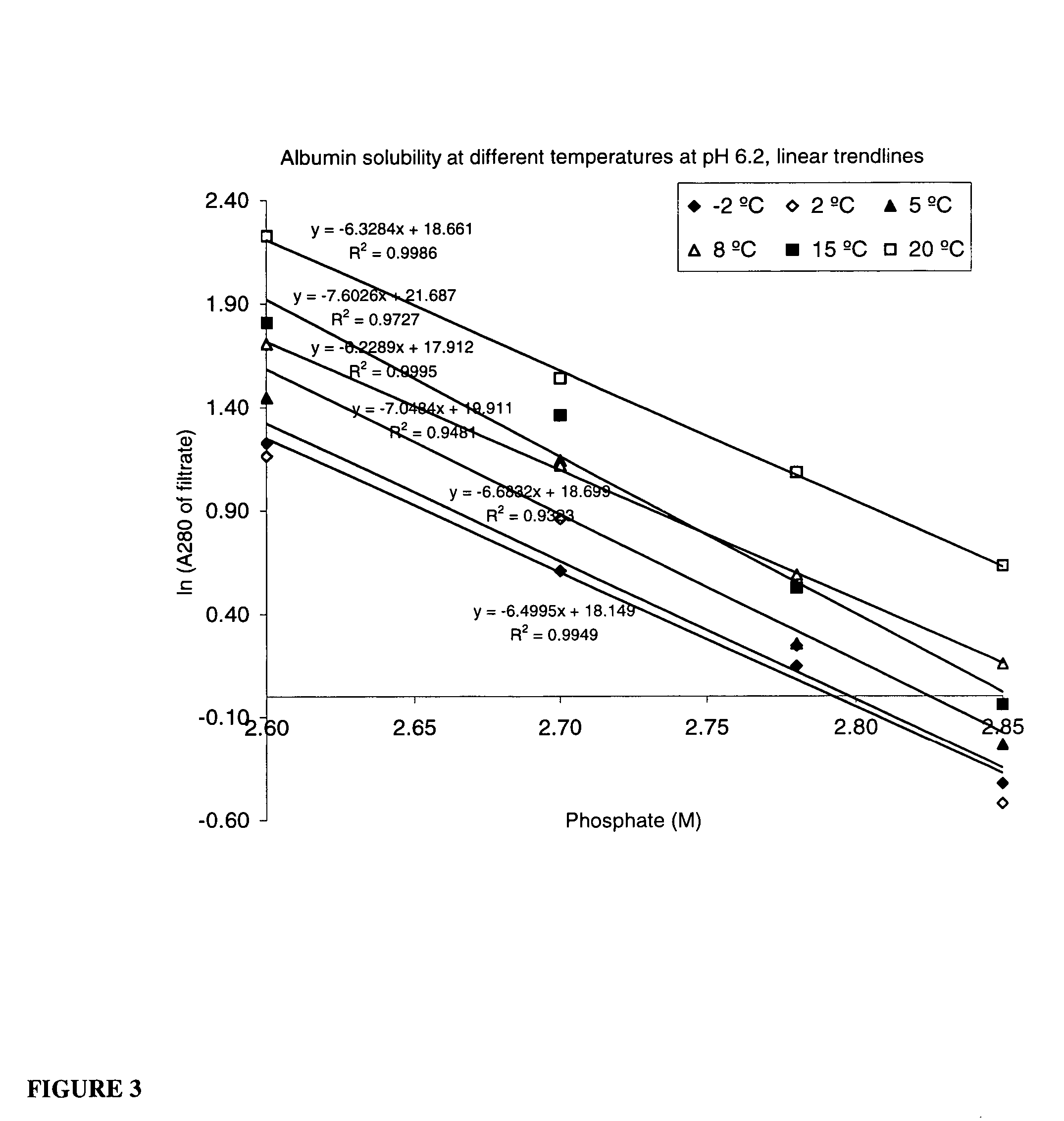
[0064] This process description is made for the crystallization of a purified albumin solution and for that purposes describes the use of only a phosphate solution only. However, according to the current invention this method can be used on impure or only partially purified starting material, as may be found from transgenic or cell culture feedstreams, with the addition of additional steps provided herein. Variations of the inventive method, for example utilizing starting solutions with significant impure material, are presented below.
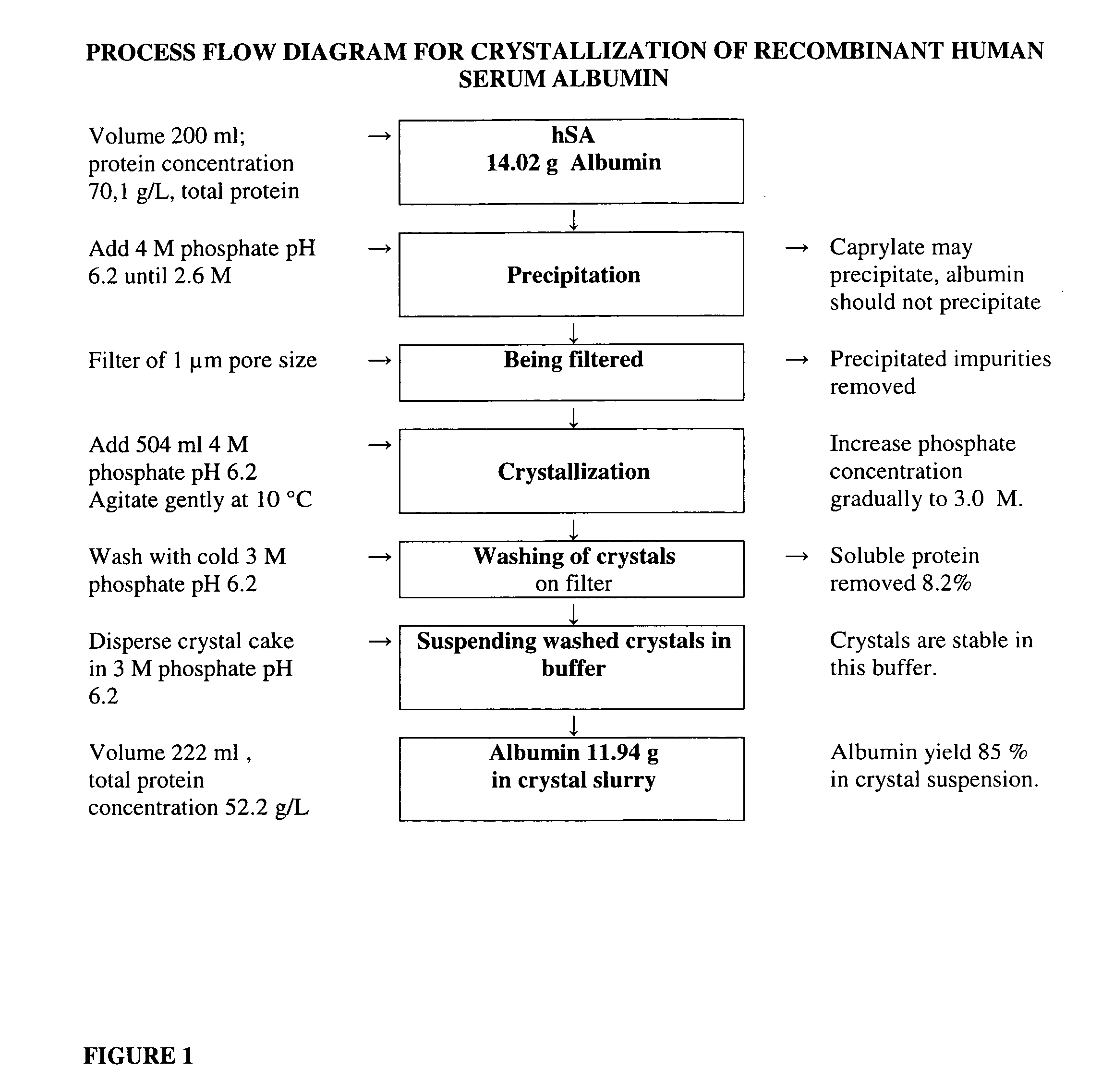
Step 1. Precipitation
[0065] Phosphate stock solution, containing 2.8 moles of NaH2PO4 and 1.2 moles of K2HPO4 dissolved in water and filled to 1.0 liter, was used in crystallization. This 4 M phosphate solution had pH 6.2 when measured after dilution to 0.5 M. Thereafter, 200 ml of a purified albumin solution was precipitated by adding 371 ml of 4.0M phosphate stock solution at a pH of 6.2. On the basis of the add...
example 2
Crystallization of Albumin with the Microdiffusion Method
[0071] Crystallization examples from 48 samples were prepared according to a hanging drop microdiffusion method known in the art. The sample solution contained purified 198 mg / ml of albumin and 3.2 mg / ml sodium caprylate. The liquid solution of albumin was prepared by mixing 3 μl of albumin solution with 3 μl of 1.8-2.3 M phosphate. The drops were allowed to equilibrate in the closed microdiffusion wells and refrigerated at 5° C. Crystals were produced in less than 24 hours according to this embodiment of the the current invention. According to the current invention, feedstreams from other source material, especially transgenic and cell culture sources, can also be utilized in conjunction with a microdiffusion hanging drop method.
[0072] The crystals were observed with microscope and photographed with digital camera. Information regarding the development of the crystals are provided on tables 19 and 20. These examples show th...
example 3
Crystallization of Albumin in Impure Starting Material
[0074] The following process description is originally made from a recombinant hSA starting material which contains much of impurities which interfere the crystallization. The first process steps are needed for removal of impurities. If more pure albumin is used, the number of steps is correspondingly reduced.
Step 1. Concentration
[0075] The starting material should be concentrated by ultrafiltration filtered as much as possible. High protein concentration will reduce the usage of phosphate and increase the yield of crystallizable rhSA. The material should also be filtered with water in the end of concentration procedure in order to reduce the salts originating from the previous process steps. Guideline for protein concentration: A280nm=150-200.
Step 2. Initial Precipitation with Phosphate
[0076] Add 2.5 volumes of 4 M phosphate (pH 6.2) into 1.5 volumes of rhSA concentrate. The final phosphate concentration at this step is 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com