Selection of cells expressing heteromeric polypeptides
a cell line and heteromeric technology, applied in the field of recombinant expression of polypeptides in animal cell culture, can solve the problems of substantially increasing the cost of recombinant, difficult to obtain a cell line expressing both chains to high levels and roughly equal amounts, etc., and achieves improved expression and efficient production of recombinant heteromeric complexes in cells.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of DHFR Complementation Vectors
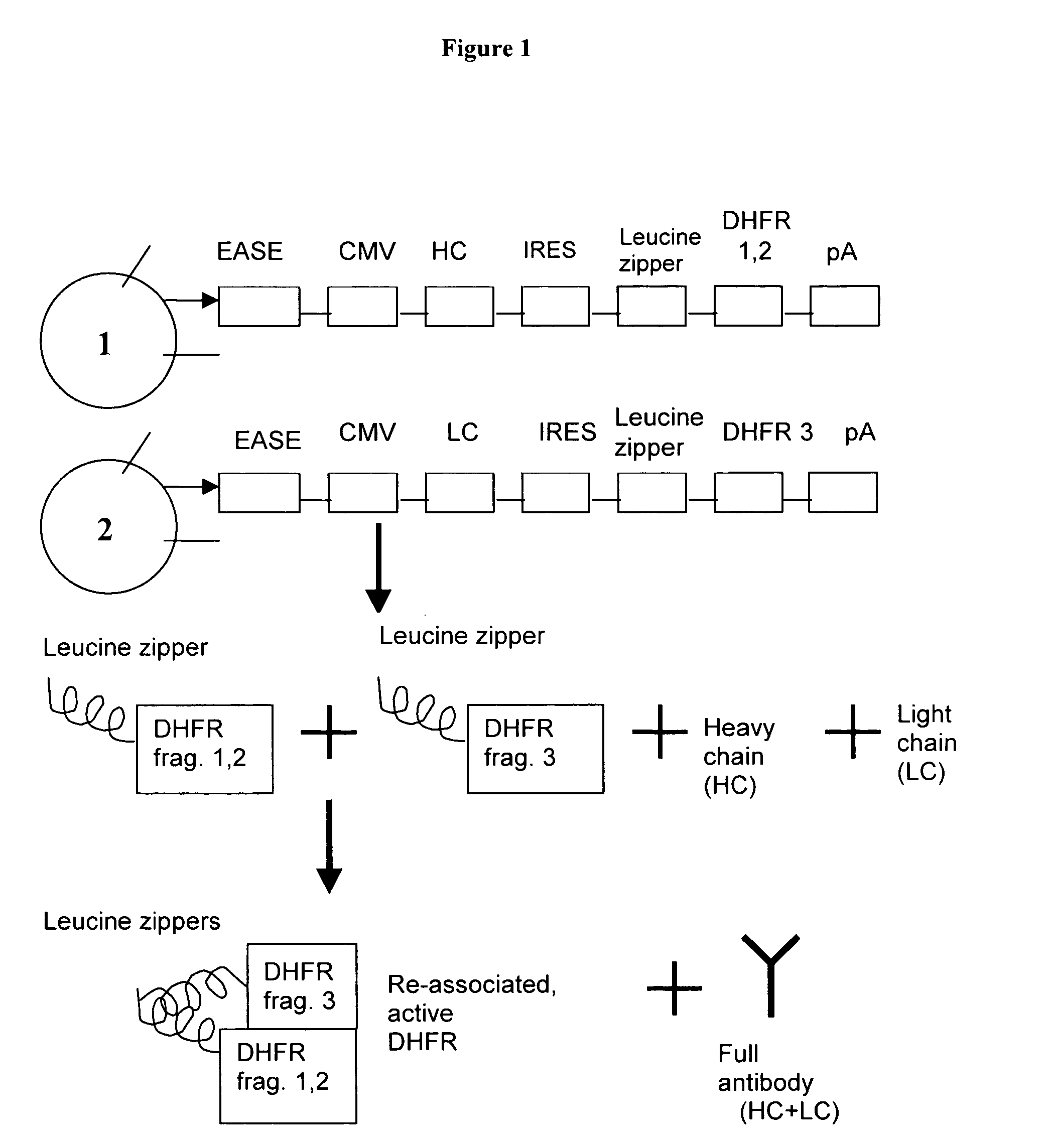
[0057] Construction of recombinant vectors expressing subunits of a selectable marker was performed as follows. Dihydrofolate reductase (DHFR) was chosen as the selectable marker to be used in the following experiments. Previous work has shown that due to its modular three-dimensional structure, DHFR can be broken into two parts and when expressed as a fusion protein having an interaction domain, the subunits can then be reassociated in a cell providing selectable activity. See FIG. 1 for a general overview of the order of the various nucleic acids described in one embodiment of the invention.
[0058] Sequential polymerase chain reaction (PCR) SOEing was utilized to generate nucleic acids suitable for cloning into expression vectors that encode a fusion of a leucine zipper interaction domain fused to a linker polypeptide fused to a subunit of DHFR. Briefly, PCR SOEing is splicing of genes by overlap extension for recombining DNA molecules ...
example 2
Construction of a Second Set of DHFR Complementation Vectors
[0076] Construction of a second set of recombinant vectors expressing subunits of a selectable marker was performed as follows. Bicistronic vectors containing the internal ribosomal entry site (IRES) are based on pED4 (Kaufman (1991), Nuc Acids Res. 19(16):4485-4490). The base vector, pDC318, is a derivative of pG2.1 (Aldrich (1998), Cytotechnology, 28:9-17) containing a truncated 600 base pair portion of the expression augmenting sequence element (EASE). pDC317 is a similar vector which contains the larger 3.6 kilobase EASE. PCR was used to fuse a GCN4 leucine zipper (LZ) and flexible linker to two separate fragments of the selectable marker dihydrofolate reductase (DHFR). The first fragment extends from amino acids 1-105 and the second fragment includes amino acids 106-187. The final PCR products were then cloned into pDC317 or pDC318 just downstream of the IRES element.
[0077] The IRES element was modified based on the ...
example 3
Transfection and Selection
[0079] Transfection of the above vectors was performed into DHFR deficient CHO cell line. Standard transfection protocols were used. Cells were incubated at 37 .degree. C. until in log phase, and transfected with an appropriate concentration of purified plasmids with 150 uL Lipofectamine (Gibco BRL) as recommended by the manufacturer. The Lipofectamine (Invitrogen) transfections were performed with a 6:6:1 ratio of either pDC321 LC:pDC322 HC:pCDNA3 (Invitrogen), pDC321 HC:pDC322 LC:pCDNA3, pDC323 LC:pDC323 HC:pCDNA3, or pDC324 HC:pDC324 LC:pCDNA3.
[0080] Initial selection was performed in shake flasks in non-DHFR selection media plus G418 with recovery of up to 70% viability, followed by selection in DHFR selection media lacking glycine, hypoxanthine and thymidine (-GHT) with recovery of up to 90% viability. Pools established following G418 and -GHT selection were exposed to 25 nM methotrexate in an attempt to amplify the antibody chains and thereby enhanc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| drug resistance | aaaaa | aaaaa |
| resistance | aaaaa | aaaaa |
| hygromycin resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com