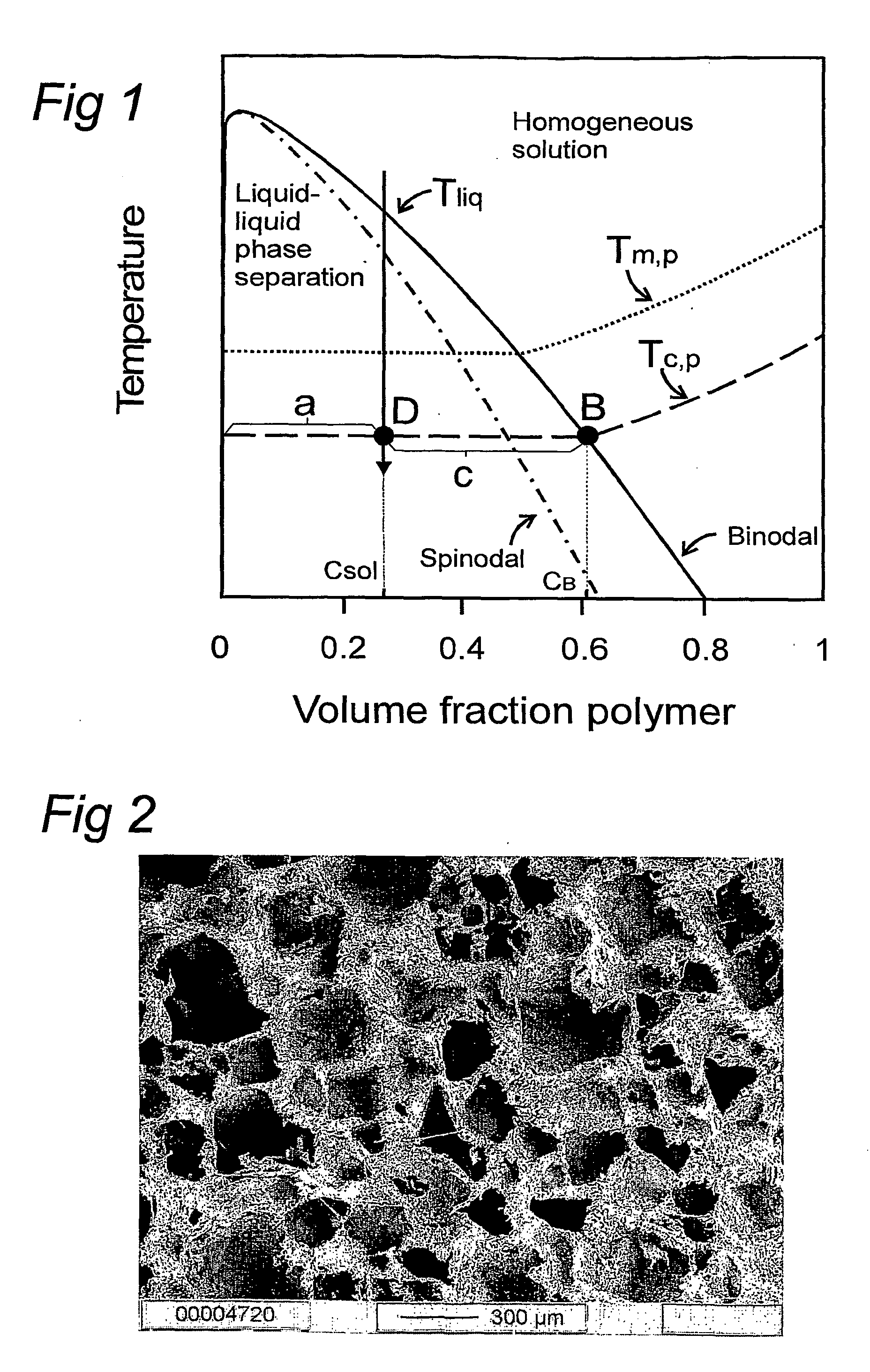
Method for the preparation of new segmented polyurethanes with high tear and tensile strengths and method for making porous scaffolds
a polyurethane and scaffold technology, applied in the field of biomedical materials, can solve the problems of unfavorable biomedical applications, unfavorable biomedical applications, and difficult processing of polyurethane ureas, and achieve the effects of less degradation of polyurethanes, and more difficult production of polyurethanes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis Polyurethane
[0143] All steps were performed under Argon atmosphere. 11.23 g (0.1246 mol) 1,4-butanediol (Aldrich, destilled from 3 Angstrom molsieves) was added to 185.86 g (1.628 mol) ε-caprolactone (Union Carbide, distilled from CaH2 under reduced pressure). This mixture was polymerized to a macrodiol 150° C. for 8 days. 50 ml (0.3229 mol) 1,4-butane diisocyanate (Bayer, distilled under reduced pressure) was added to 56.10 g (0.03546 mol) of the obtained macrodiol. The 1,4-butane diisocyanate and the poly(ε-caprolactone) prepolymer were reacted at 80° C. for 3.5 hours to obtain a macrodiisocyanate. The surplus diisocyanate, was distilled off. 60.69 g (0.0326 mol) of the obtained macrodiisocyanate was chain extended with 2.91 g (0.0323 mol) 1,4-butanediol at 80° C. until the intrinsic viscosity was at least 0.8 dl / g.
example 2
Mechanical Properties
[0144] In table 1 tear and tensile strengths of polyurethanes made according to example 1 poly(ε-caprolactone) segments of different length are compared to polyurethanes made according to prior art method where the poly(ε-caprolactone) diol segment was made using 0.08 wt. % stannous octoate as a catalyst (wt catalyst / wt polymer). Spaans used 0.1 wt. % catalyst for the preparation of the macrodiol, which is equal to 0.08 wt. % catalyst to the polymer for a macrodiol length of 2000 g / mol) (Spaans et. al. Polymer Bulletin, 41, 131-138, 1998, C. J. Spaans, Biomedical Polyurethanes Based On: 1,4-Butanediisocyanate: An Exploratory Study. 2000 PhD Thesis ISBN 90-367-1232-7, chapter 2).
[0145] Polymer films were by made according to the procedure described by Spaans (Polymer Bulletin, 4.1., 131-138, 1998) by dissolving the polymer in 1,4-dioxane at 100° C. at a concentration of 6% (w / w). The solvent was removed at 70° C. Last traces of solvent were removed under reduce...
example 3
Tear Strength as Function of Intrinsic Viscosity
[0150] The tear strength, determined according the procedure described in example 2, of polyurethanes made according to example 1 as a function of intrinsic viscosity was determined. The results are shown in table 2.
TABLE 2IntrinsicTear strengthPolyurethaneviscosity (dl / g)(kJ / m2)PU1000a0.395PU1000a0.76211PU1000a0.84213PU1000a1.18211
apolymer made according to the invention. No catalyst was used for the synthesis of the macrodiol. The macrodiisocyanate chain extended with 1,4-butanediol
[0151] It is shown that the tear strength increases with intrinsic viscosity in the range of 0.39 to 0.76 dl / g. Above 0.76 g / mol, the tear strength is constant.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com