Fuel electrode of solid polymer electrolyte fuel cell
a fuel cell and solid polymer technology, applied in the direction of active material electrodes, cell components, electrochemical generators, etc., can solve the problem of increasing the potential of the fuel electrode, and achieve the effect of maintaining performance and reducing density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
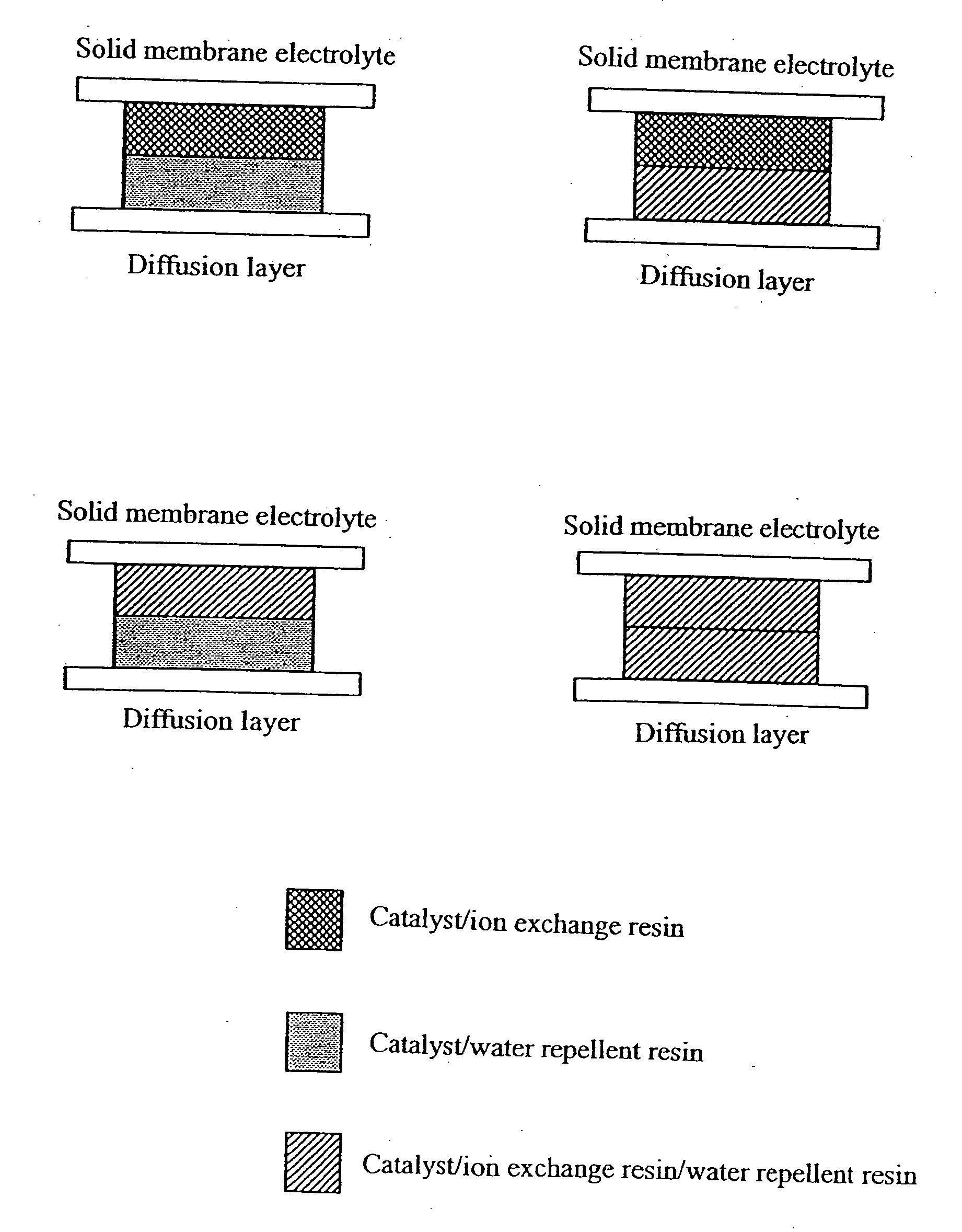
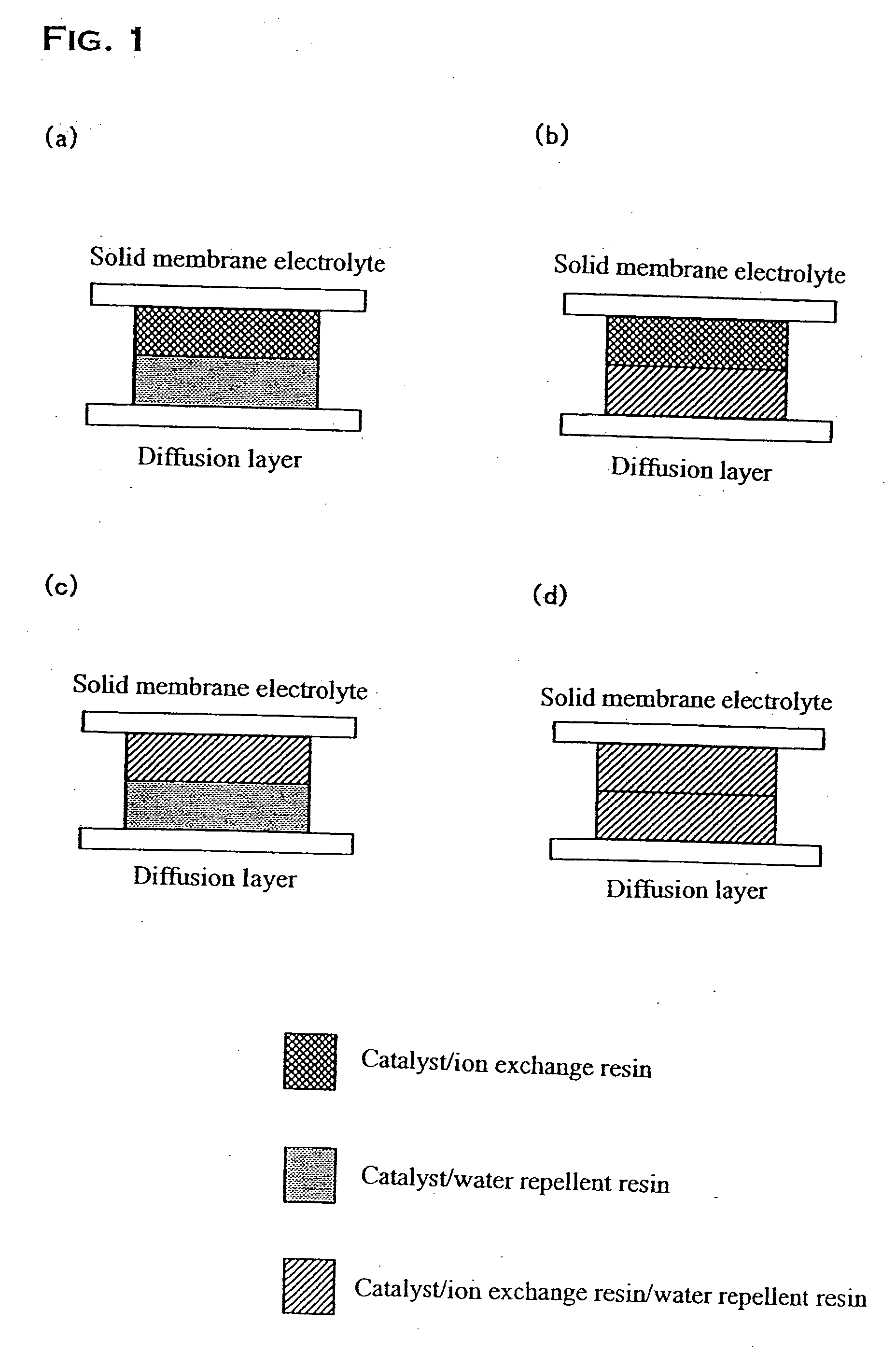
[0038] In the present embodiment, the fuel electrode corresponding to (a) in FIG. 1 was produced. First, 1 g of fine powders of the resin prepared by spray-drying a 5% solution of the above described ion exchange resin and 2 g of the above described platinum-ruthenium catalyst were added to 25 mL of an aqueous solution of 1-propanol, and they were mixed for 50 minutes with a ball mill, preparing a catalyst paste. Next, the catalyst paste was applied by printing on the surface of a polymer electrolyte membrane (Nafion 112) such that the loading of platinum is 0.2 mg / cm2 to form the reaction layer.
[0039] On the other hand for the water decomposition layer, 1 g of the above described fine powders of the water repellent resin and 1.7 g of the above described platinum catalyst were added to 25 mL of an aqueous solution of 1-propanol, and they were mixed for 50 minutes with a ball mill, preparing a catalyst paste. Next, the catalyst paste was applied by printing on the surface of a diffu...
second embodiment
[0041] In the present embodiment, the fuel electrode corresponding to (b) in FIG. 1 was produced. The reaction layer was produced in a manner similar to First Embodiment. On the other hand, an ion exchange resin was further added to the water decomposition layer in the present invention. Specifically, 1 g of fine powders of the ion exchange resin was further added when producing the catalyst paste for the water decomposition layer, other processes being similar to First Embodiment. The produced reaction layer and water decomposition layer were bonded to form the fuel electrode by a method similar to First Embodiment.
third embodiment
[0042] In the present embodiment, the fuel electrode corresponding to (c) in FIG. 1 was produced. A water repellent resin was further added to the reaction layer of this embodiment. Specifically, 1 g of fine powders of the water repellent resin was further added when producing the catalyst paste for the reaction layer, other processes being similar to First Embodiment. On the other hand, the water decomposition layer was produced in a manner similar to First Embodiment. The produced reaction layer and water decomposition layer were bonded to form the fuel electrode by a method similar to First Embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
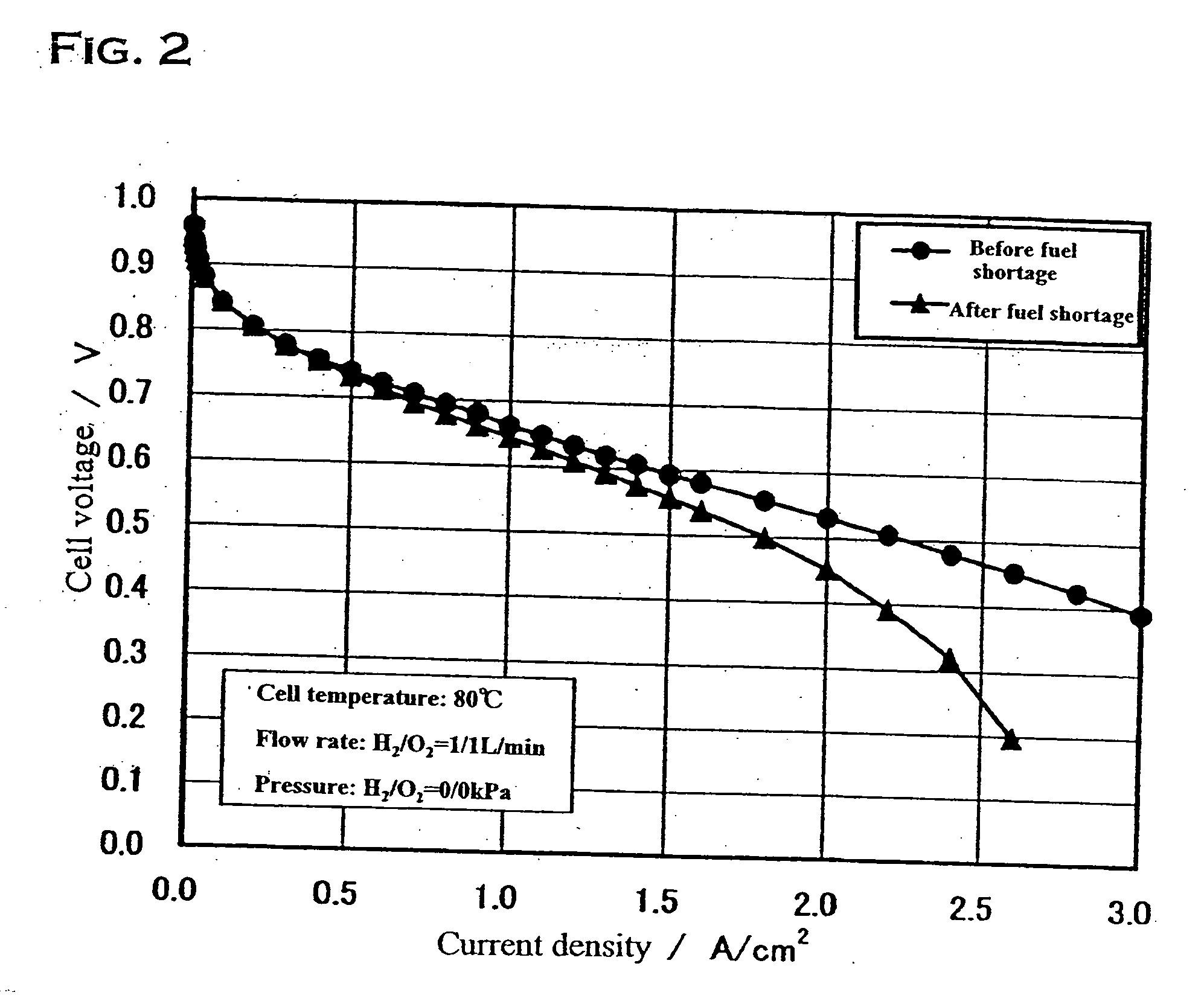
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com