Methods of use for 2,5-dihydroxybenzene sulfonic acid compounds for the treatment of cancer, rosacea and psoriasis
a technology of benzene sulfonic acid and 2,5-dihydroxybenzene sulfonic acid, which is applied in the direction of biocide, drug composition, immunological disorders, etc., can solve the problems of no cure for psoriasis, poor treatment effect of patients with advanced solid tumors, etc., and achieve the effect of improving the efficacy of chemotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2,5-Dihydroxybenzene Sulfonic Acid Potentiates Chemotherapy
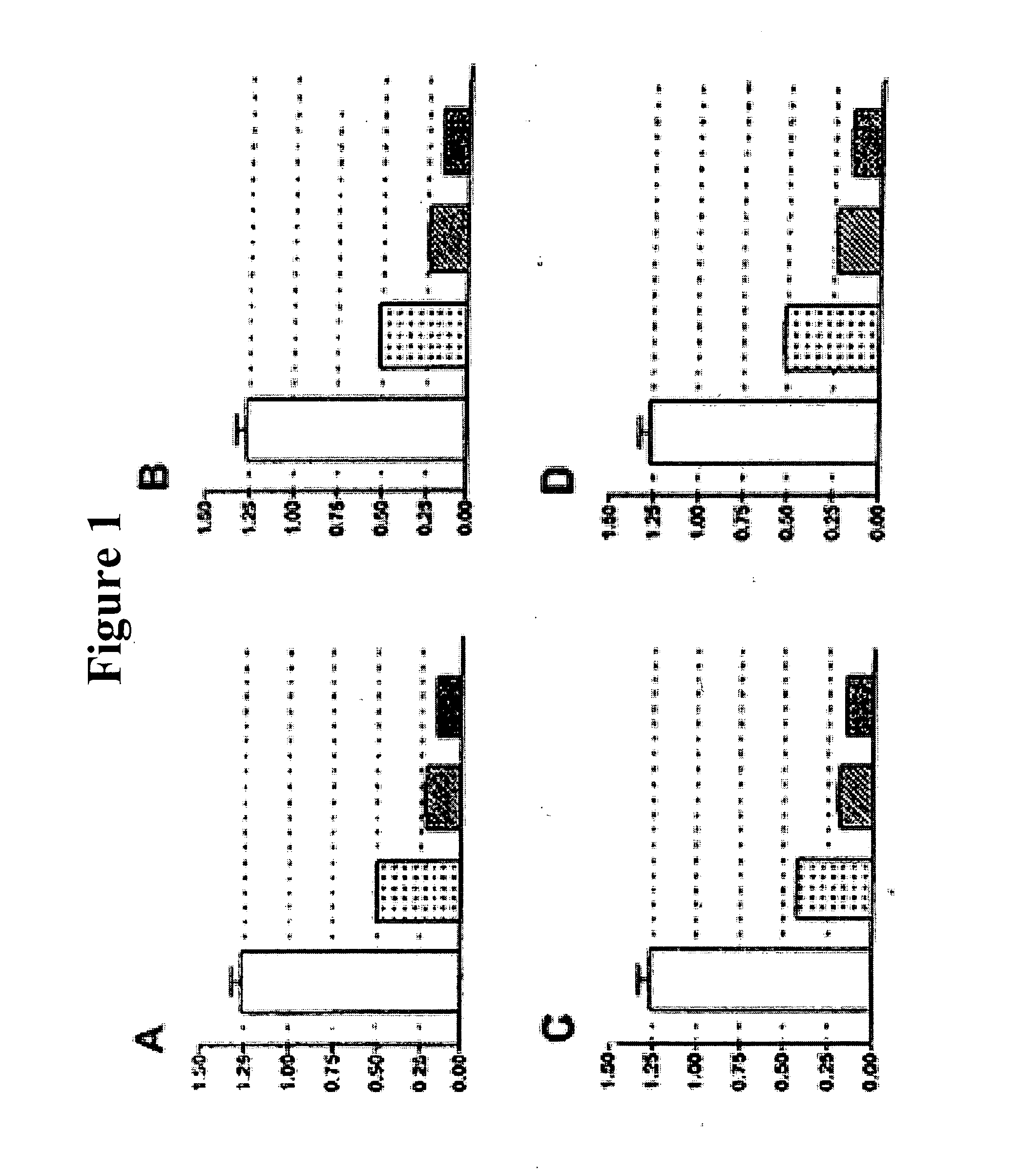
[0135] C6 cells cultured in vitro under the same conditions as described in PCT Application No. 2005 / 077352. 1. 1×103 cells per well were cultured in 24-well plates. Three types of treatment were conducted: a) 24 hours after seeding, the cells were separately treated with each of the following medicines; cisplatin (5 μg / ml), vincristine (0.1 μg / ml), paclitaxel (5 μg / ml) and 5-fluorouracil (100 g / ml); b) 24 hours after seeding, the cells were treated with a combination of 2,5-dihydroxybenzene sulfonic acid (100 μM) and each one of the following agents; cisplatin (5 μg / ml) vincristine (0.1 μg / ml), paclitaxel (5 μg / ml) and 5-fluorouracil (100 μg / ml); c) at the time of seeding (Day 0), the cells were pre-treated with 2,5-dihydroxybenzene sulfonic acid (100 μM). The next day the cultures were also treated with each one of the following agents: cisplatin (5 μg / ml) vincristine (0.1 μg / ml), paclitaxel (5 μg / ml) and 5-fluorouracil (...
example 2
2,5-Dihydroxybenzene Sulfonate Increases the Chemo Sensitivity in Rat C6 Glioma Cells
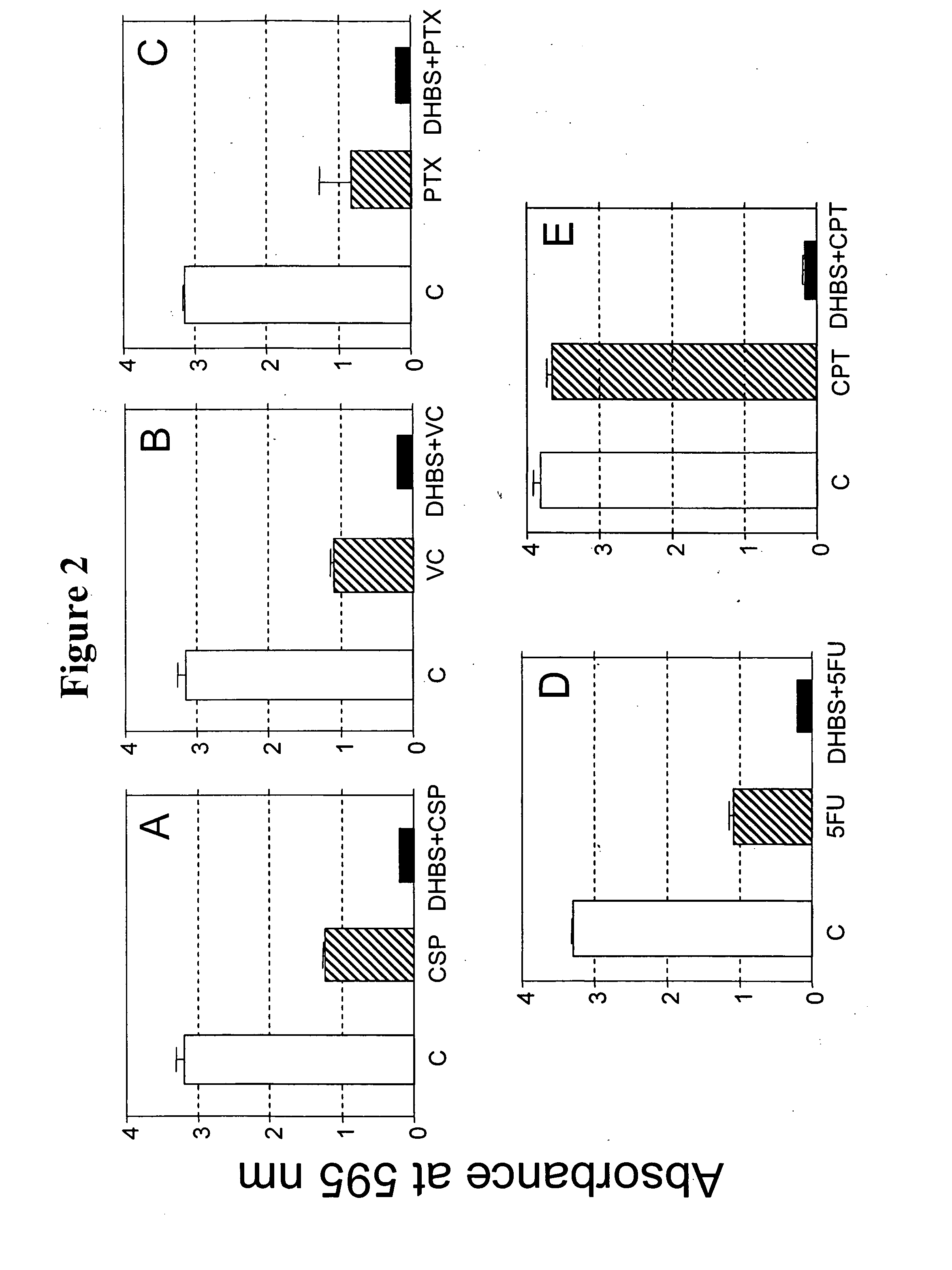
[0138] Cultures of rat glioma C6 cells were grown as described in Cuevas P et al. Neurol. Res., 27: 797-800 (2005). Briefly, cells were grown as adherent cells in Dulbecco modified Eagle medium supplemented with 1% (v / v) fatal bovine serum, 10 μg / ml streptomycin and 10 units / ml penicillin (DMEM). Tumour cells were set up in 24-well cell culture plates, at a density of 10,000 cells per well and incubated at 37° C. in a humidified chamber with 5% CO2. Cells were either treated with 2,5-dihydroxybenzene sulfonate (100 μM in DMEM) or with DMEM (controls). Eighty hours after the addition of 2,5-dihydroxybenzene sulfonate, the cells were either treated or not treated with the cytostatic agents, cisplatin (5 μg / ml in DMEM), vincristine (0.5 μg / ml in DMEM), paclitaxel (5 μg / ml in DMEM), 5-fluorouracil (100 μg / ml in DMEM) and irinotecan (CPT-11; 0.1 μg / ml in DMEM). After an additional two days the prolifera...
example 3
2,5-Dihydroxybenzene Sulfonate Increases the Efficacy of Irinotecan (CPT-11) in Rat Glioma
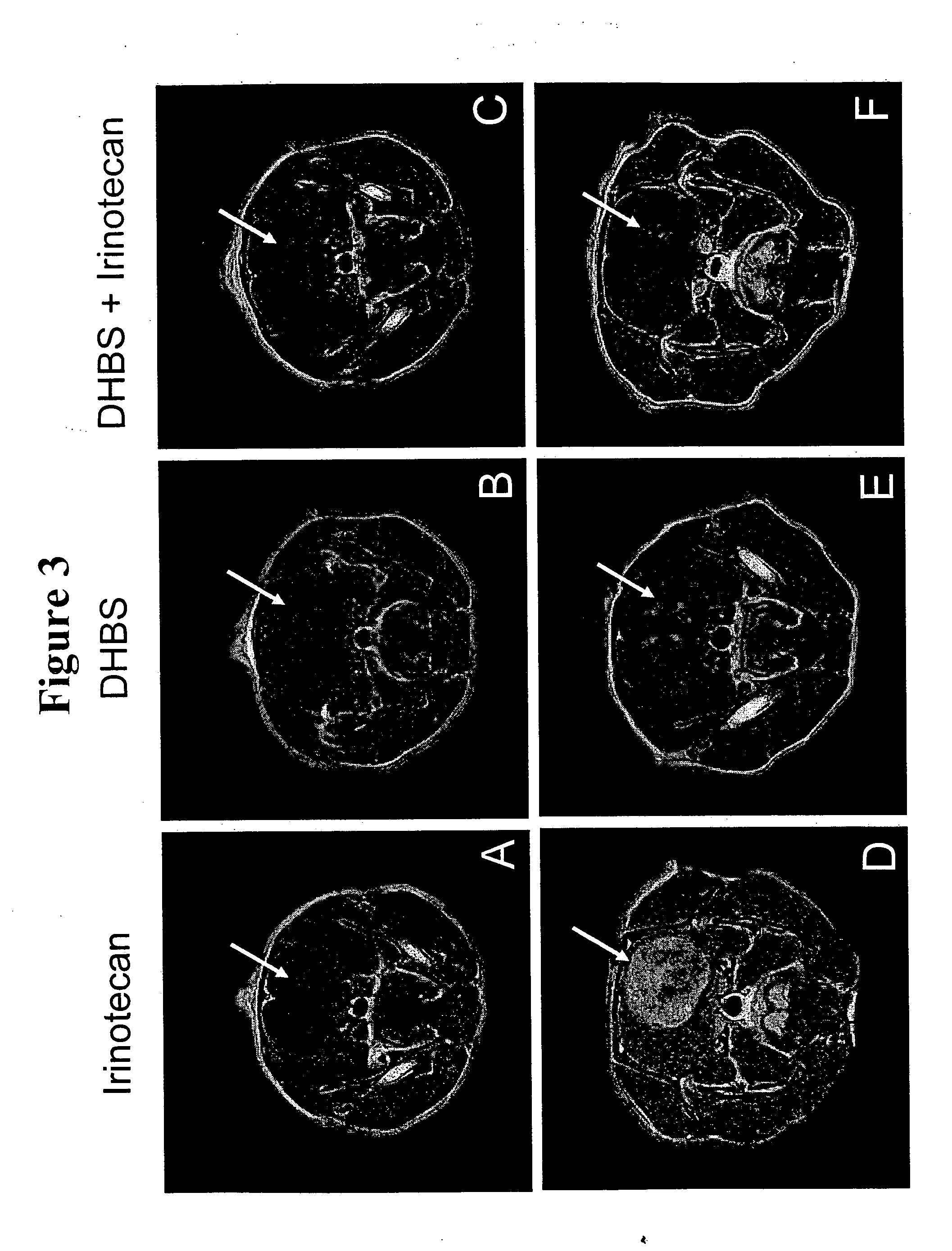
[0140] Cultures of rat glioma C6 cells were grown as described in Cuevas P et al. Neurol. Res., 27: 797-800 (2005). Confluent C6 cells cultured in 75 cm2 flasks were detached and implanted into the left frontal lobe of anaesthetized rats using a stereotactic device. The implantation of the 106 glioma cells was accomplished by means of a Hamilton syringe coupled to a glass pipette (40-60 μm in diameter) through a small orifice performed in the cranium by a stainless steel needle. FIG. 3, panels A, B and C shows the magnetic resonance image of the rat brain two weeks after implantation. Ten days after the existence of a tumour was confirmed, rats were randomly assigned to receive or not receive 2,5-dihydroxybenzene sulfonate (200 mg / kg / day in saline; i.p) throughout the period of observation. Two days after starting the treatment with 2,5-dihydroxybenzene sulfonate, the rats were treated with ir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
| Antimicrobial properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com