Neurotherapeutic composition and method therefor
a neuropsychiatric and composition technology, applied in the direction of peptide/protein ingredients, peptide sources, metabolic disorders, etc., to achieve the effects of improving learning and memory, reducing impulsivity and violence, and reducing disorientation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental examples
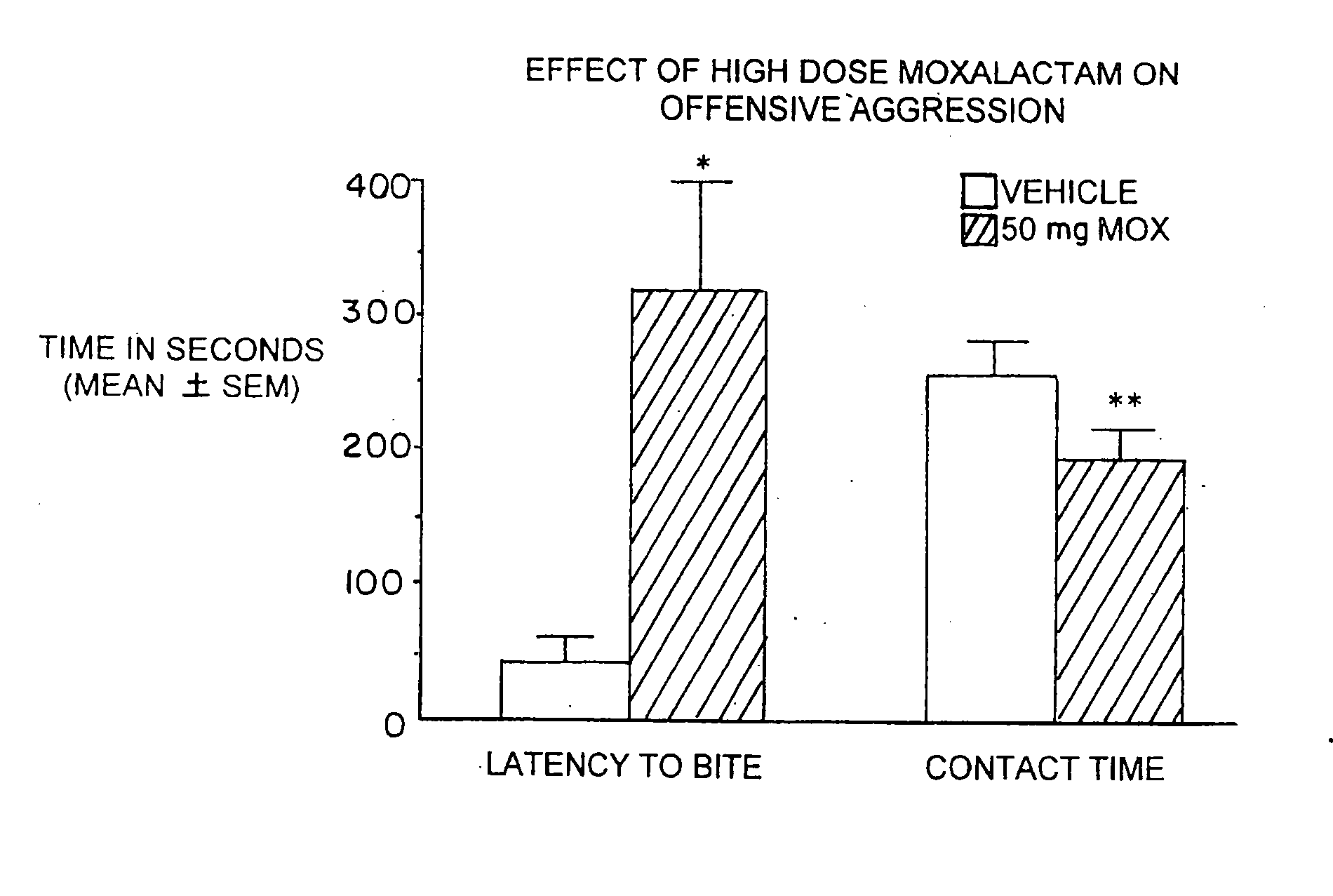
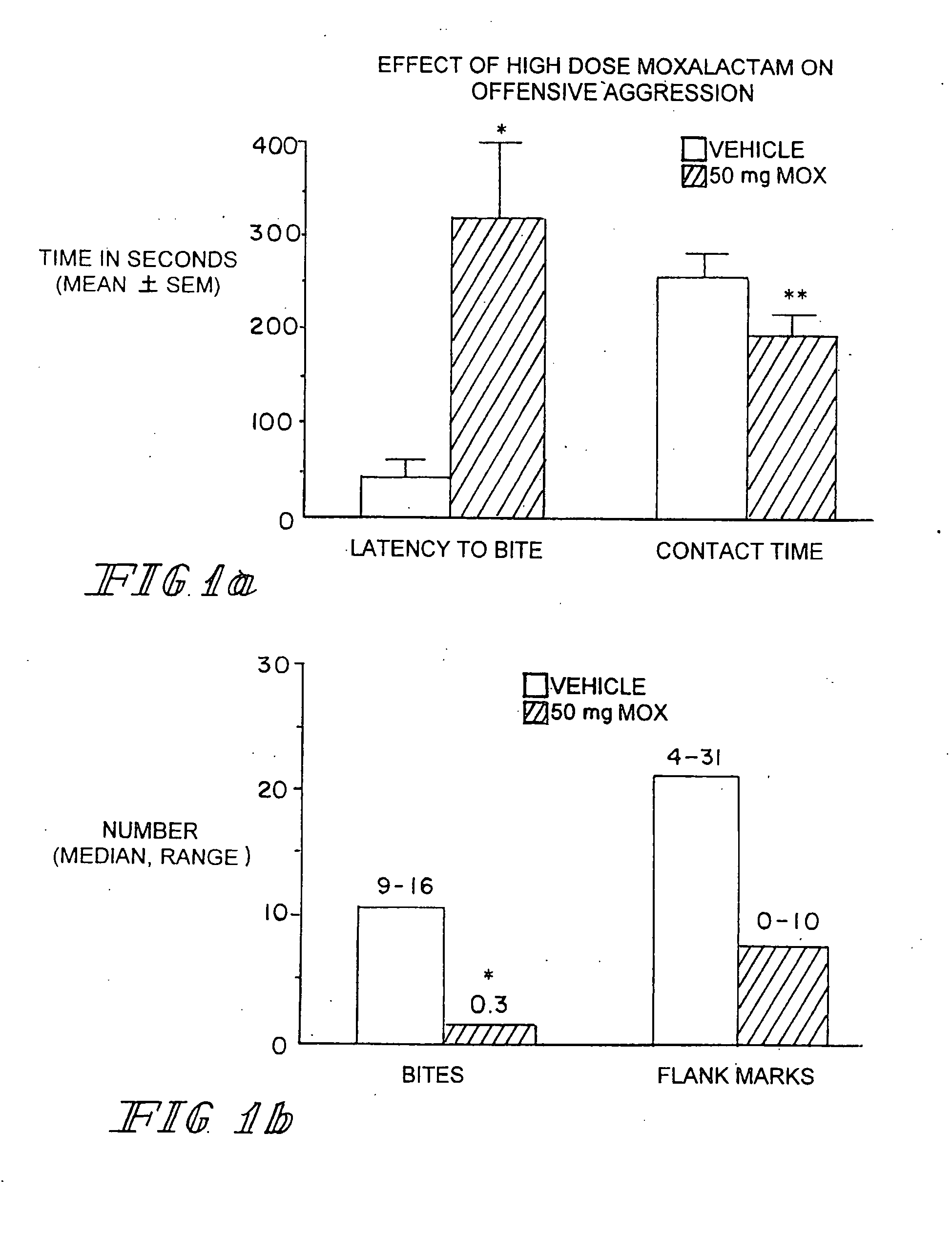
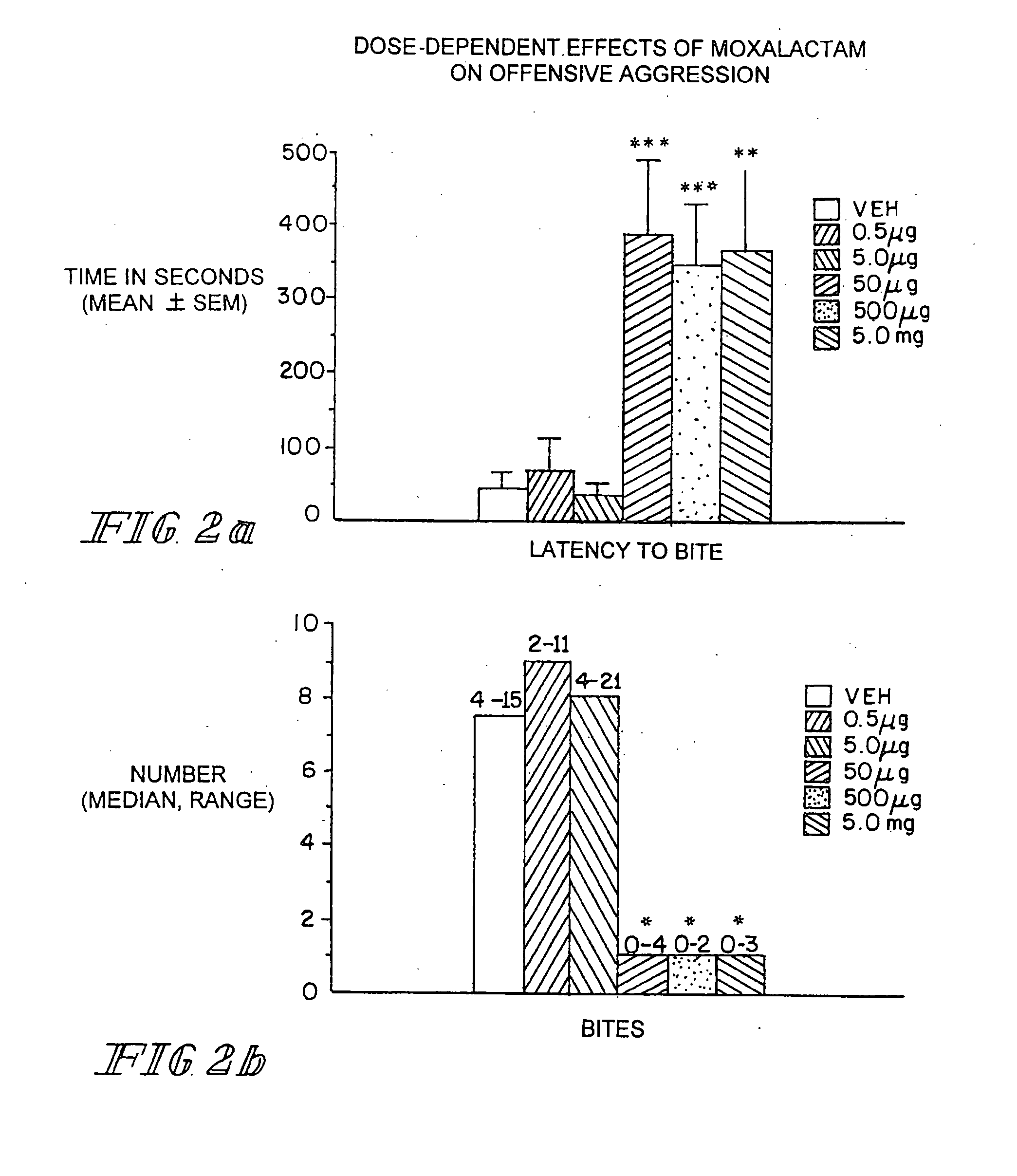
[0055] Marketed in 1981-1982 moxalactam (Mox) was employed widely in the world as a third-generation cephalosporin-like antibiotic. Clinical efficacy and safety were evaluated in over 2200 patents with bacterial infections (Jackson et al. 1986). Of the 260 patents treated with Mox for gram-negative meningitis, 241 (93%) showed satisfactory response to antibiotic therapy. Patents were treated with 4 g of Mox every 8 hrs for 2-3 weeks. Peak plasma concentrations occur within an one hr after IM injection with an elimination half-life of 2.3 hrs. There is no accumulation with multiple injections occurring at 8-12 hr intervals. Moxalactam can penetrate the blood brain barrier. Cerebrospinal fluid (CSF) levels of Mox range from 25-39 μg / ml following a 2.0 g IV dose of drug. The CSF concentration as a percentage of serum concentration is estimated to be 20%. The D isomer has antibacterial activity and has a greater unbound fraction to plasma protein that the L isomer.
Behavioral Studies wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com