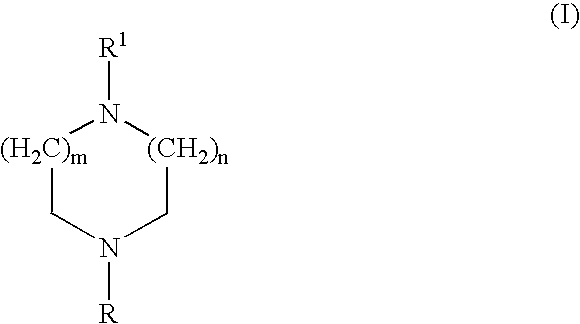
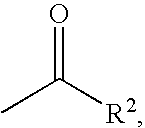
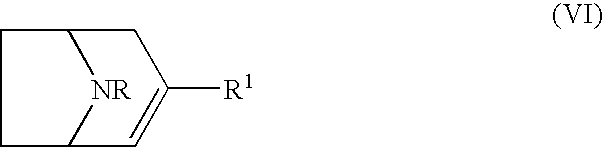Treatment of affective disorders by the combined action of a nicotinic receptor agonist and a monoaminergic substance
a technology monoaminergic substance, which is applied in the field of combined action of nicotinic acetylcholine receptor agonist and monoaminergic substance, can solve the problems of window of vulnerability during which the patient might not be able to comply with therapy, and achieve the effect of increasing the success rate and accelerating the onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparatory Example
General
[0159] All reactions involving air sensitive reagents or intermediates were performed under nitrogen and in anhydrous solvents. Magnesium sulphate was used as drying agent in the workup-procedures and solvents were evaporated under reduced pressure.
Method A
1-(5-Bromo-3-pyridyl)-piperazine Fumaric Acid Salt (Compound 1A1)
[0160] A mixture of 3,5-dibromopyridine (100 g, 422 mmol) and piperazine (72.7 g, 844 mmol) was stirred at 130° C. for 100 hours. Water (400 ml) was added and the pH was adjusted to 7 by adding hydrochloric acid (4 M). The aqueous phase was washed three times with ethyl acetate (3×600 ml). The aqueous mixture was made alkaline by adding sodium hydroxide (200 ml, 4 M). The aqueous mixture was extracted four times with diethyl ether (4×500 ml). The product was isolated as free base.. Yield 44 g (43%). The corresponding salt was obtained by addition of a diethyl ether and methanol mixture (9:1) saturated with fumaric acid. Mp 185.0° C.
1...
example 2
Preparatory Example
General
[0192] All reactions involving air sensitive reagents or intermediates were performed under nitrogen and in anhydrous solvents. Magnesium sulphate was used as drying agent in the workup-procedures and solvents were evaporated under reduced pressure.
9-Methyl-3,9-diazabicyclo-[4.2.1]-nonane
[0193] was prepared according to [Michaels R J & Zaugg H E; J. Org. Chem. 1960 25 637].
(±) 3-(2-Naphthalyl)-9-methyl-3,9-diazabicyclo-[4.2.1]-nonane Fumaric Acid Salt (Compound 2A)
[0194] A mixture of 2-bromonaphtalene (5.0 g; 24.1 mmol), 9-Methyl-3,9-diazabicyclo[4.2.1]-nonane (3.38 g; 24.1 mmol) and palladacycle (0.045 g; 0.048 mmol) [Angew. Chem. Int. Ed. Engl. 1995 34 1844] was stirred for two days at 150° C. Sodium hydroxide (50 ml; 1 M) was added at room temperature. The mixture was extracted with diethyl ether (2×100 ml). Chromatography on silica gel with dichloromethane, methanol and conc. ammonia (89:10:1) gave the title compound. The corresponding salt was ...
example 3
Mouse Forced Swim Test
[0206] Systemic administration of a classical antidepressant (Desipramine) induces an increase in the forced swim distance performed by mice in a small water pool. The Forced Swim Test therefore is considered predictive of a potential antidepressant pharmacological effect.
[0207] Female NMRI mice (of 20-25 g) are habituated to the laboratory (a 12 hour light / dark cycle) for at least 16 hours before the experiment.
[0208] 30 minutes after s.c. injection of either vehicle or drug, the mouse is placed in a glass beaker (d=16 cm; 5000 ml) with water (24° C.) up till 10 cm from the top edge. For the subsequent 6 minutes the duration of immobility defined as swim speed less than 2.5 cm / minute, and forced swim distance defined as distance swum with a speed above 5 cm / minute, is recorded. There are 8 mice in each group.
[0209] The activity of the mouse is tracked by the View Point system for the measurement of distance, immobility, and speed.
[0210...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com