Apparatus and method for easing use of a spectrophotometric based noninvasive analyzer
a non-invasive, spectrophotometric technology, applied in the field of apparatus and methods for easing the use of a spectrophotometric based non-invasive analyzer, can solve the problems of inconvenient use, inconvenient use, and inability to accurately measure glucose, so as to improve detection accuracy, increase detection ease, and increase the accuracy of near-ir based non-invasive glucose determination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
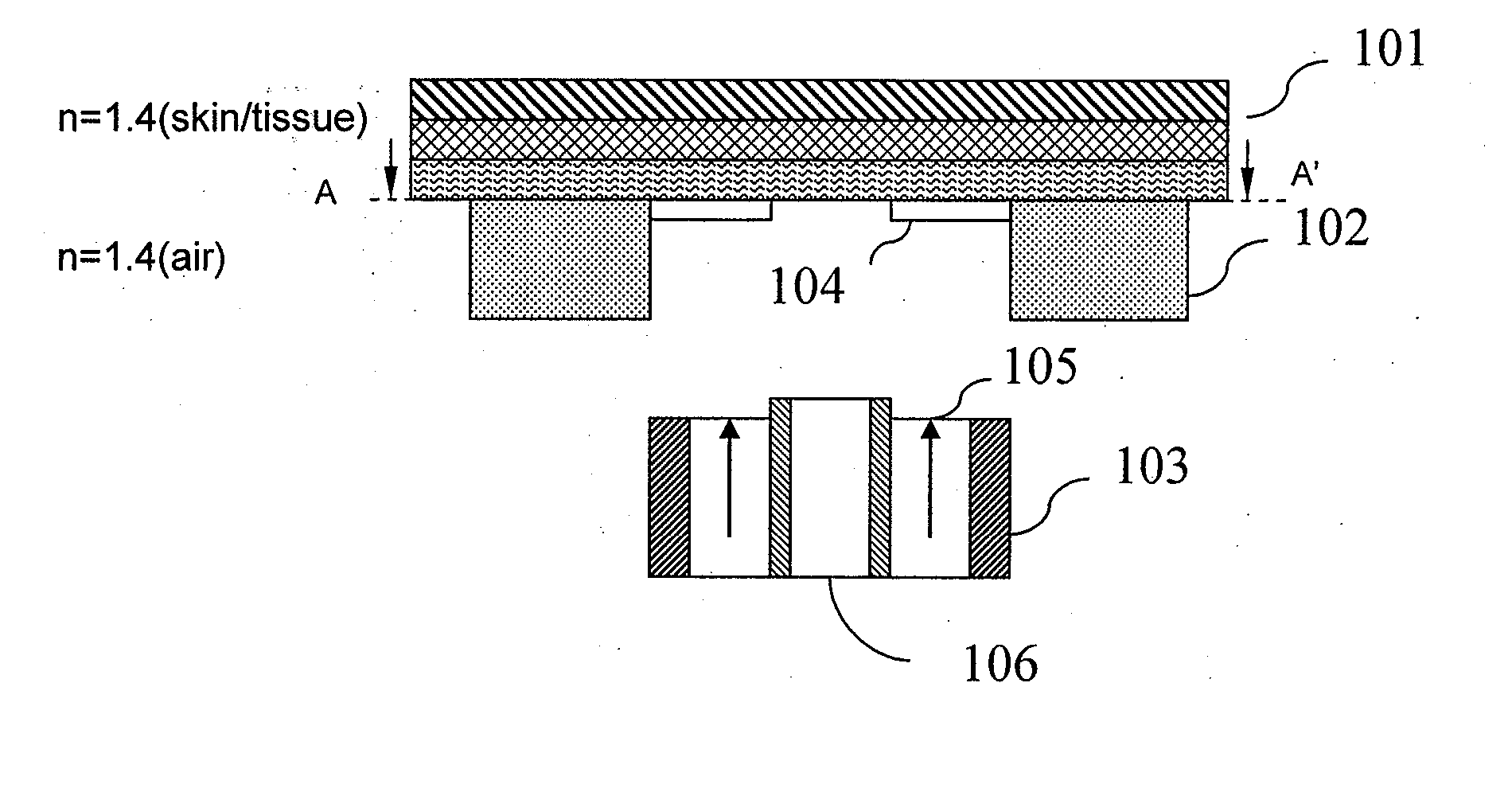
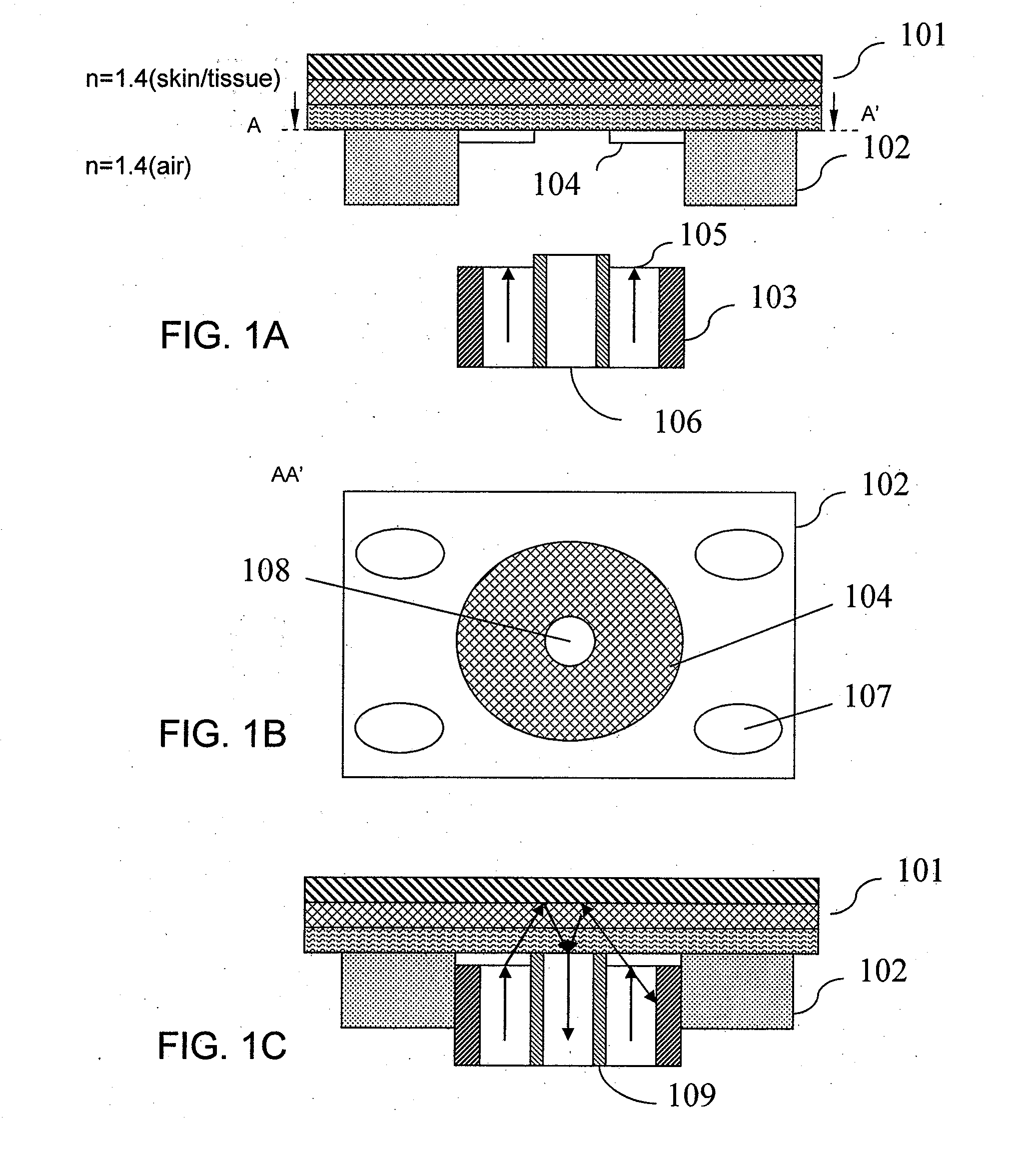
[0061]FIG. 1A is a schematic diagram showing the front, cross-sectional view of a piece of sampling skin, a placement guide in contact with the sampling skin, and an optical probe which has not yet been coupled with the guide according to one embodiment of the invention. In FIG. 1A, skin 101 is schematically represented in layers. These layers include the stratum corneum, epidermis, dermis, and adipose tissue, as well as underlying structures, such as muscle. Each of these layers includes many sub-layers and structures. The guide includes a mount 102, also called a guide lock, that is semi-permanently attached to a skin surface. The mount 102 may be in any shape, such as substantially rectangular, circular, oval, and polygonal. The guide includes an aperture which is defined by the mount 102. The aperture may be in any shape, such as substantially rectangular, circular, oval, triangular, hexagonal, and polygonal. The guide may be attached for one or more subsequent glucose determina...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com