Methods for Prediction of Clinical Outcome to Epidermal Growth Factor Receptor Inhibitors by Cancer Patients
a technology of epidermal growth factor receptor and clinical outcome, applied in the field of biomarkers, methods and assay kits, can solve the problems of high mortality rate, difficult treatment or relatively unresponsiveness, and standard two-drug combination generating considerable toxicities, and requiring intravenous administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
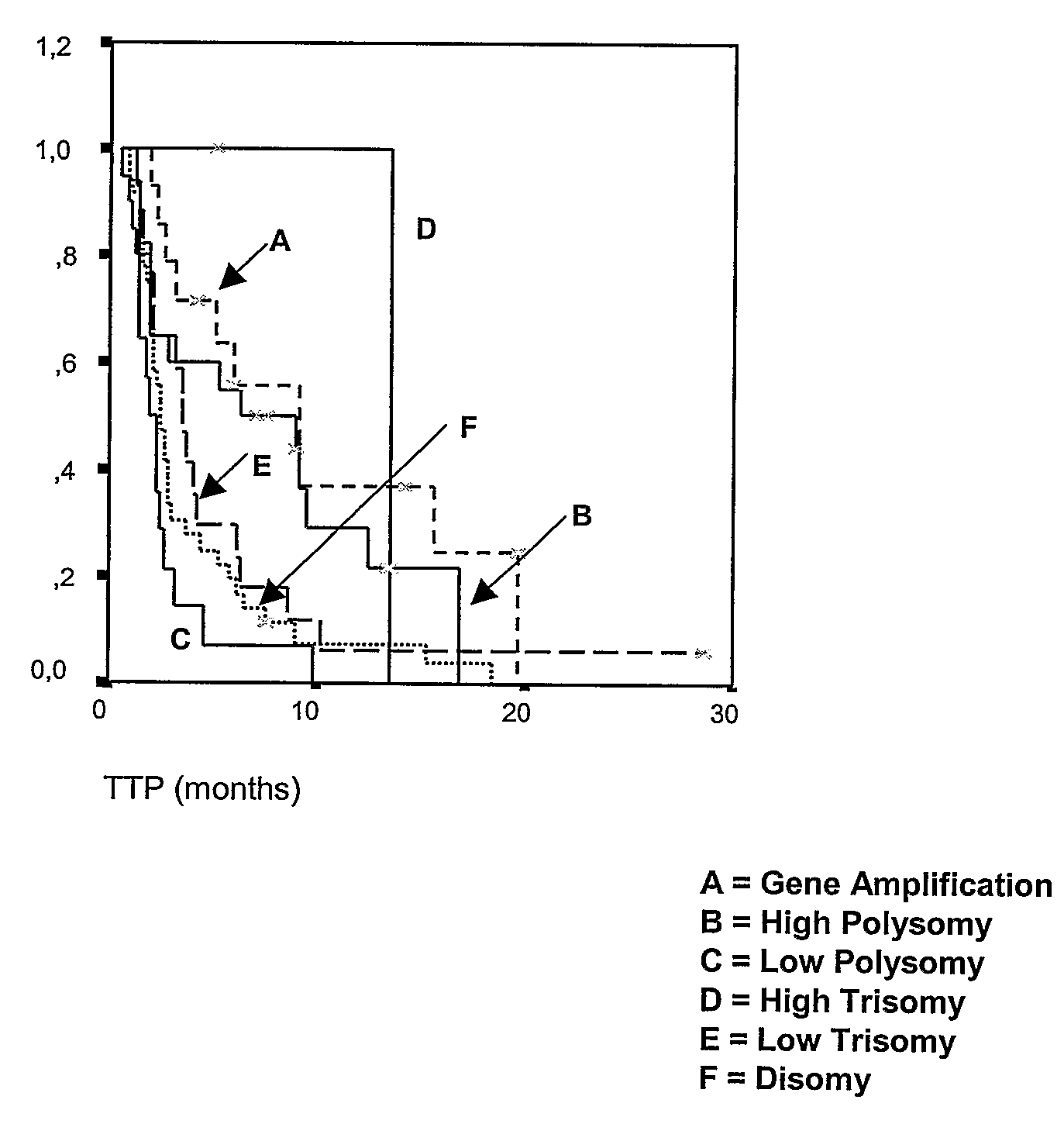
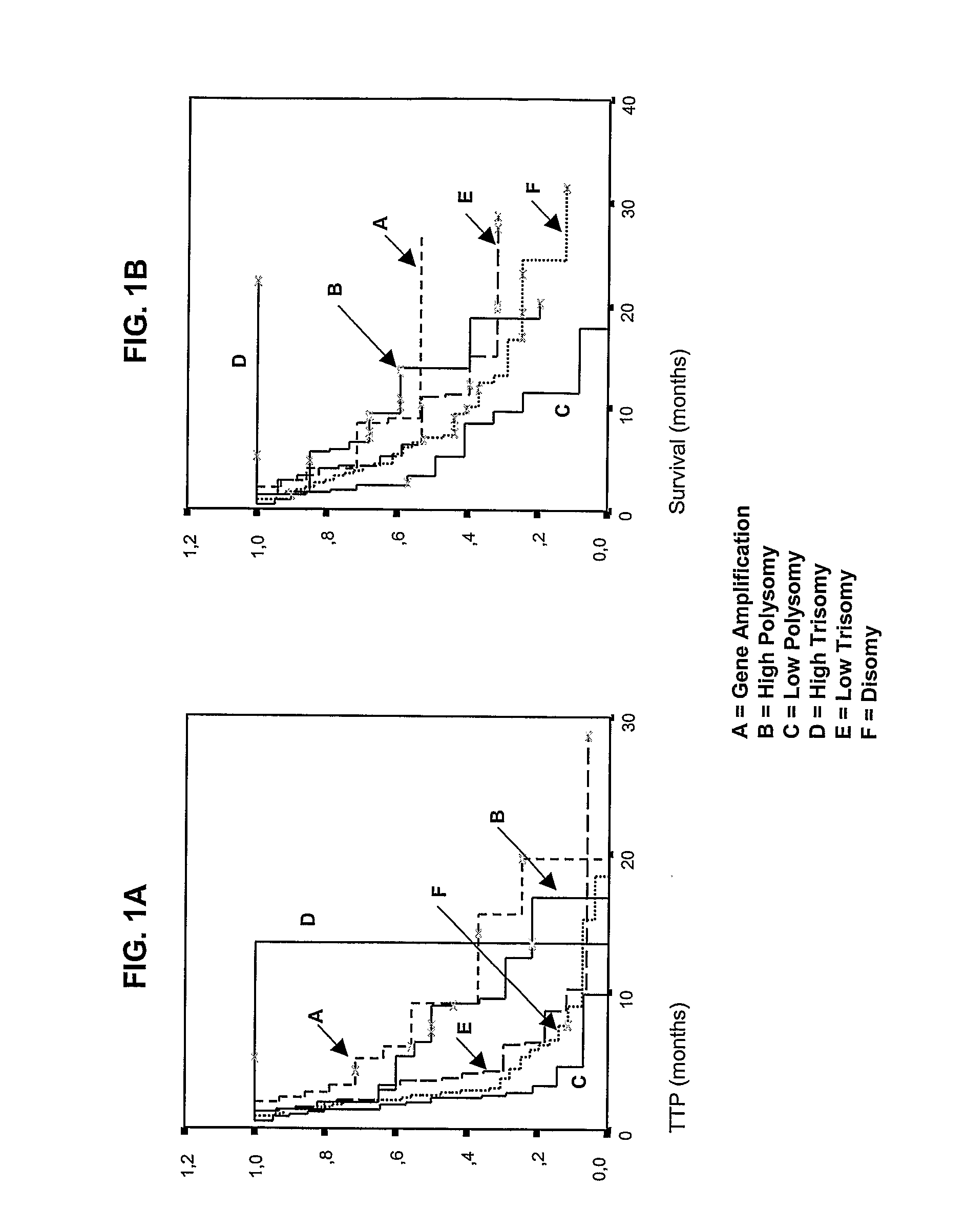
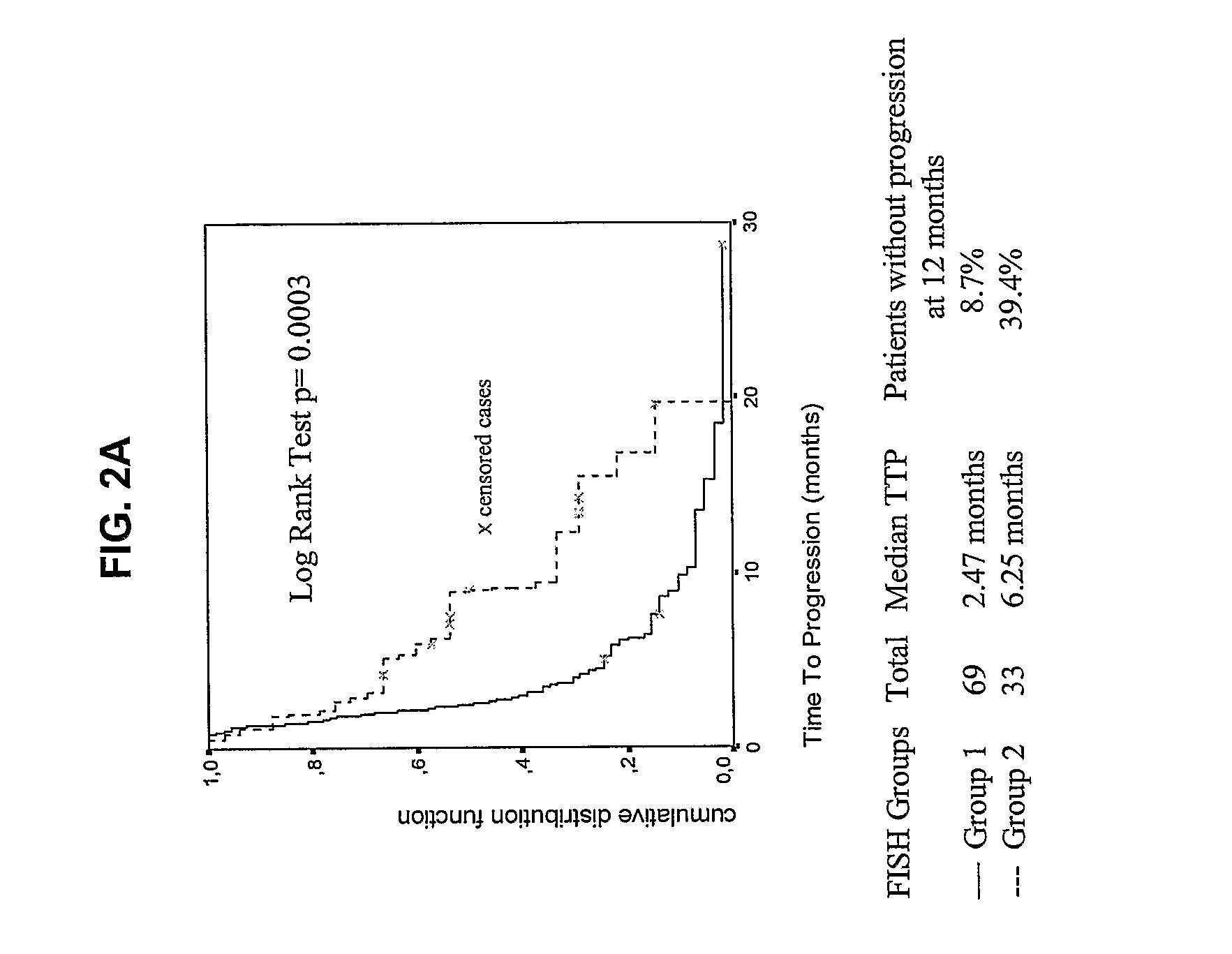
[0107]The following example demonstrates the use of detection of EGFR gene amplification and polysomy to predict treatment outcome of NSCLC tumors to EGFR inhibitors (based on the study of an Italian cohort).
Methods
Patient Selection and Study Design
[0108]Patients for this study were accrued in three Italian institutions: the Bellaria Hospital, (Bologna), the Scientific Institute University Hospital San Raffaele (Milano), and the Policlinico Monteluce (Perugia). Eligibility included histologically confirmed NSCLC patients with measurable, locally advanced or metastatic disease, who had progressed or relapsed after chemotherapy, and patients ineligible for chemotherapy because they were elderly, had poor performance status, or comorbid medical condition. Before trial inclusion, smoking status was assessed and patients were classified as never, former (smoking cessation>6 months prior to trial inclusion), or current smokers (cessation<6 months before trial inclusion or active smoker). ...
example 2
[0127]The following example demonstrates the use of detection of EGFR gene amplification and polysomy to predict treatment outcome of patients with BAC tumors to EGFR inhibitors (based on the SWOG cohort).
[0128]Bronchioalveolar carcinoma (BAC) subtypes of NSCLC are characterized by unique pathologic, radiographic, and clinical features (Travis et al., 1999), and appears to be increasing in incidence, particularly in younger non-smoking women (Barsky et al., 1994; Furak et al., 2003). BAC and adenocarcinoma with BAC features have been reported to be particularly sensitive to EGFR tyrosine kinase inhibitors, with response rates of 25-30% (Miller et al., 2003) and prolonged survival in a subset of patients. The inventors and colleagues have previously reported the efficacy of gefitinib in a large cohort of advanced stage BAC patients treated on a prospective clinical trial of the Southwest Oncology Group (S0126) (Gandara et al., 2004). Since archival tumor tissue was collected from the...
example 3
[0146]The following example demonstrates the use of EGFR protein expression, phosphorylated AKT expression, and the combination of these markers with EGFR gene copy numbers and EGFR mutation to predict outcome to EGFR inhibitor therapy in NSCLC patients (Italian cohort).
Methods
Patient Selection and Study Design
[0147]Patients included in this study were accrued from a prospective study of gefitinib (Cappuzzo et al., 2004, J. Natl. Cancer Inst.) and the Expanded Access Study of gefitinib conducted at Bellaria Hospital (Bologna), Scientific Institute University Hospital San Raffaele (Milano), and Policlinico Monteluce (Perugia). Complete clinical information and tissue blocks were available from 80 out of 106 patients enrolled in the Akt clinical trial (Cappuzzo et al., ibid.), and from an additional 22 patients in the Expanded Access Study who were treated consecutively at the end of the Akt study and followed in the same way as patients in the Akt trial. These studies were approved b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting temperatures | aaaaa | aaaaa |
| melting temperature | aaaaa | aaaaa |
| melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com