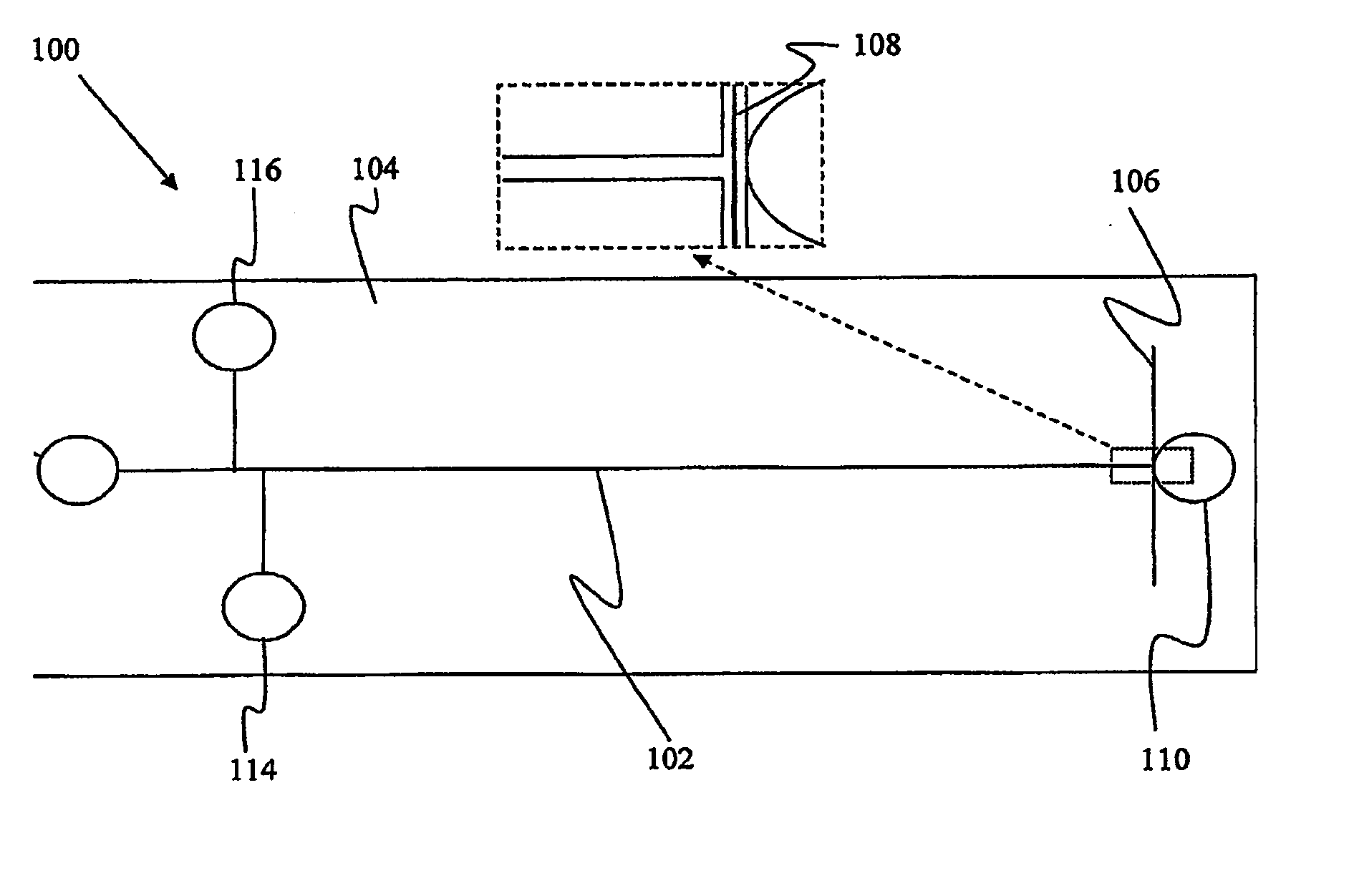
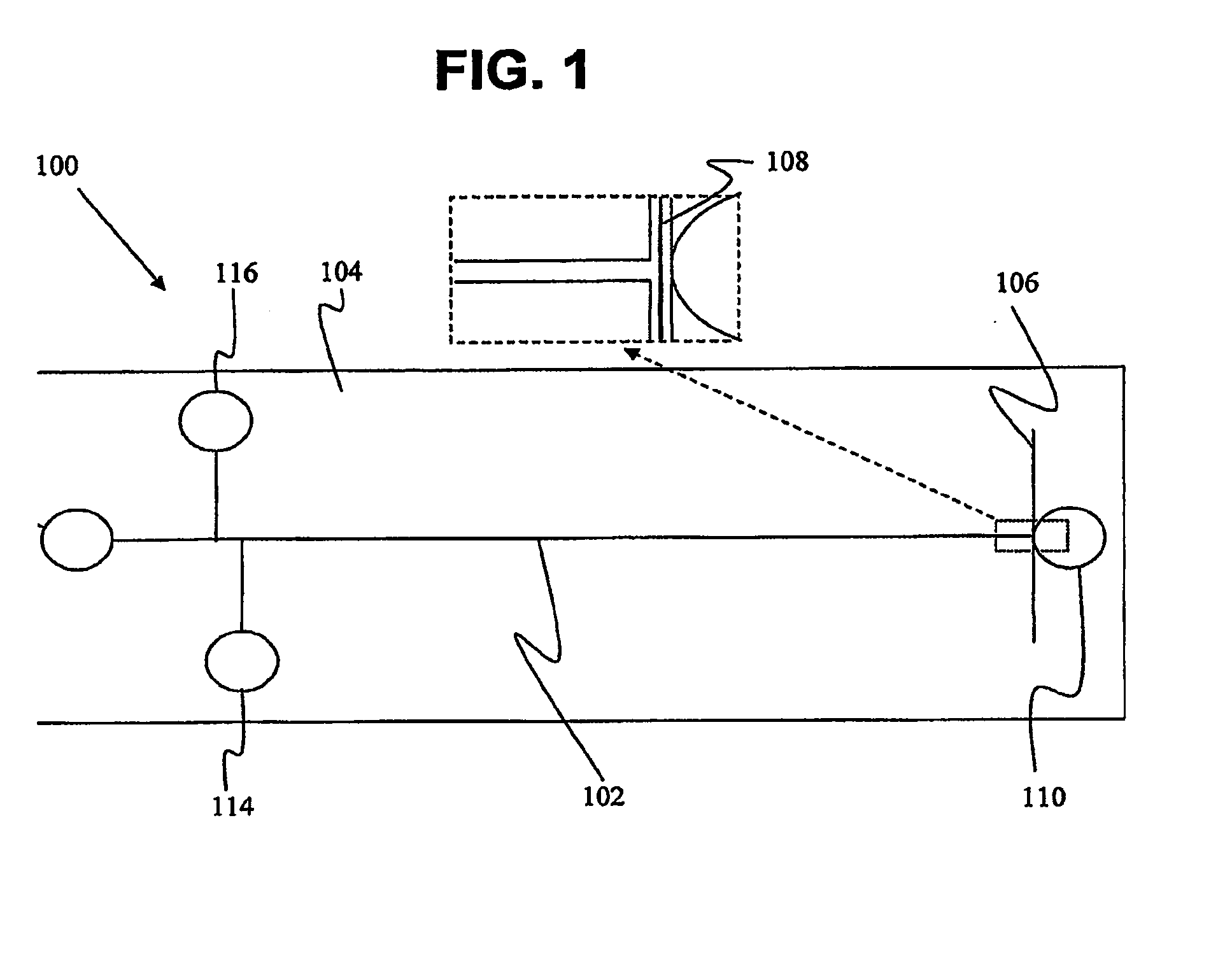
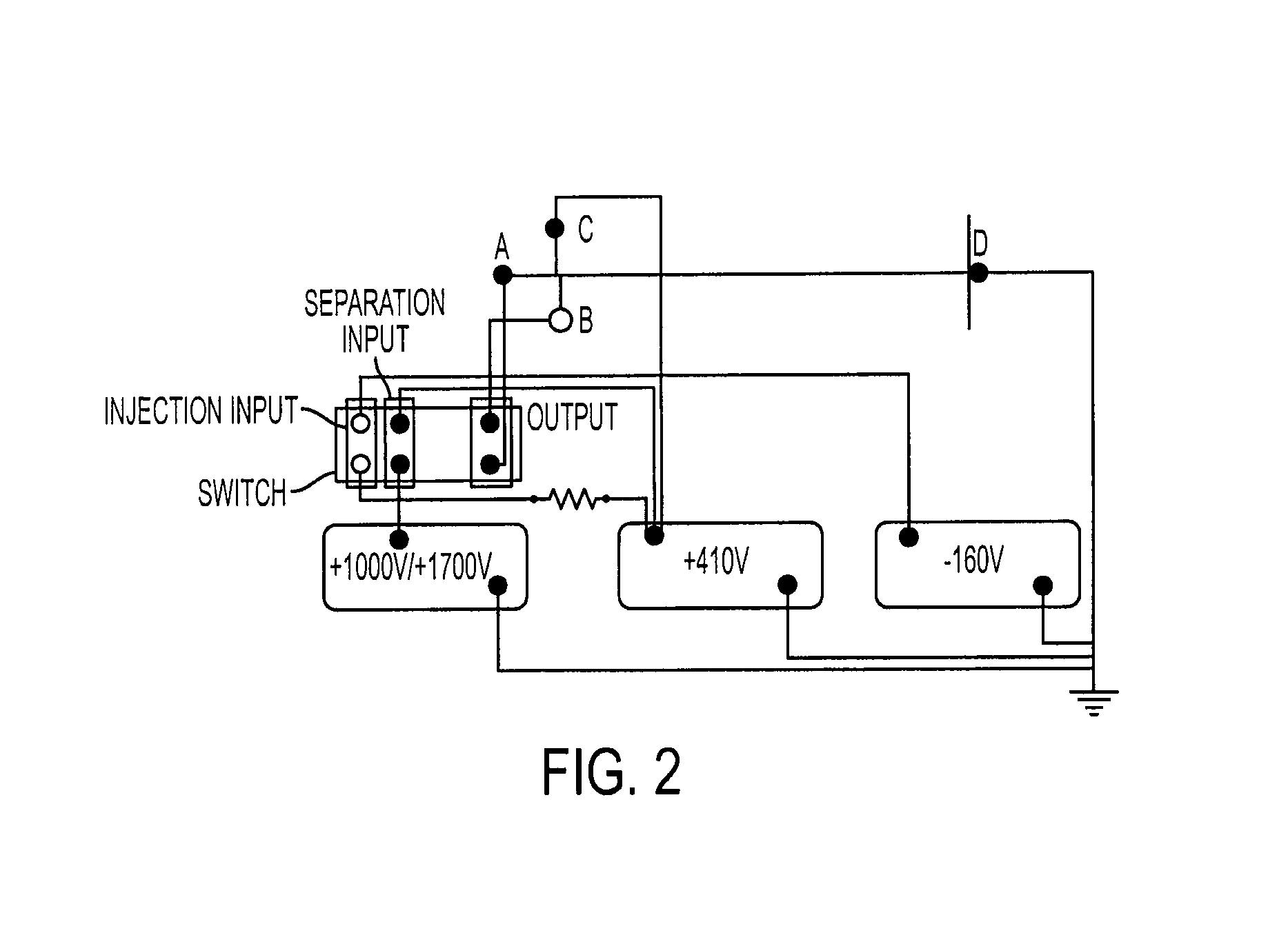
Non-Fluidic Microdetection Device and Uses Thereof
a non-fluidic microdetection and detection device technology, applied in the field of microchips, can solve problems such as optical instrumentation cos
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
[0063]A solution of 1 .μM fluorescein, prepared in the running electrolyte, was used to follow the injection. For the present experiments, injection times of 10 seconds were used to ensure plug homogeneity because at the slowest electroosmotic flow (EOF) conditions, the double-T was filled in 7 seconds. Injection times are dependent on the sample and the chip and may vary from about 1 second to about 1 minute.
example ii
Analysis of Carbohydrates
[0064]It is well known that activation barriers for oxidation of some compounds may be decreased at a clean Au electrode, see LaCourse, W. R., Pulsed Electrochemical Detection in High-Performance Liquid Chromatography, Wiley J. & Sons: New York, 1997, the entire contents and disclosure of which is hereby incorporated by reference. These surfaces stabilize free-radical oxidation products by adsorption and, thereby, may promote faradaic reactions. Once a clean surface is obtained, a potential should be chosen in order to maximize the electrode response. The effect of the detection potential on the signal was analyzed between −0.3 to +1.1V for glucose (GLU), lactose (LAC) and sucrose (SUC) as shown in FIG. 5. As may be seen, the peak current increases as the potential increases until a maximum in the signal is obtained. The following current decrease observed at higher potentials may be explained as the result of the formation of oxide on the working electrode ...
example iii
Analysis of Amino Acids
[0070]The direct detection of unlabeled amino acids has the potential to simplify quantification of these important analytes. The effect of the potential applied to the working electrode was analyzed between −0.3 and +1.1 V for the three amino acids. As was observed for carbohydrates, the peak current increased as the potential increased until a maximum in the signal was obtained at around 0.7 V. Since similar profiles were found between the selected amino acids, 0.7 V was chosen as a suitable detection potential. As part of the detection potential determination, a higher cleaning potential was selected when sulfur-containing compounds were injected in order to decrease the peak tailing produced by the high interaction between the sulfur group and the Au.
[0071]The electrolyte conditions may not only affect the separation process but also the detection step. It was previously reported that the adsorption of the amines through the free pair of electrons may be r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle | aaaaa | aaaaa |
| 90° angle | aaaaa | aaaaa |
| electrical potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com