High Pressure Processing of Metal Ion Lactoferrin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
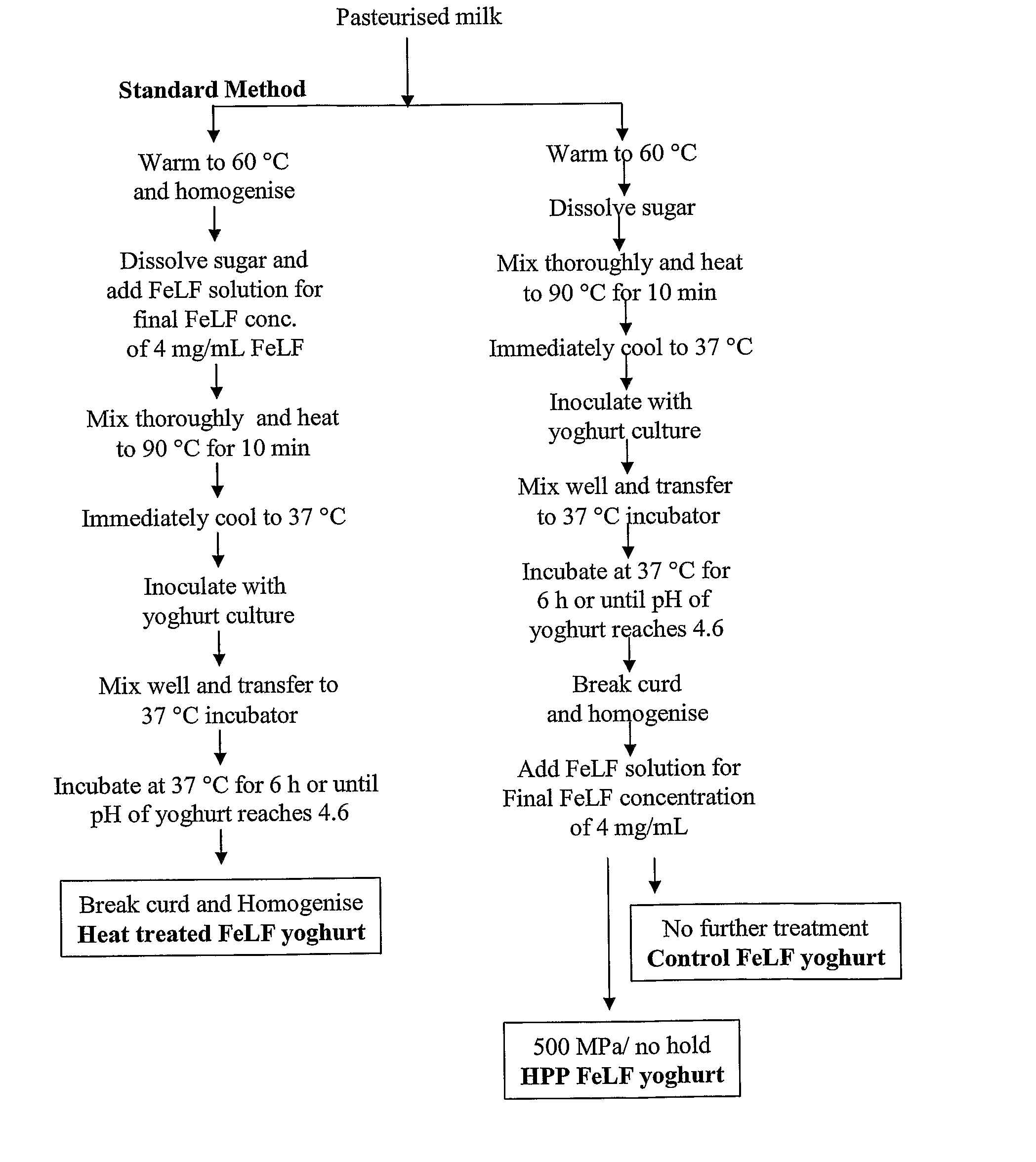
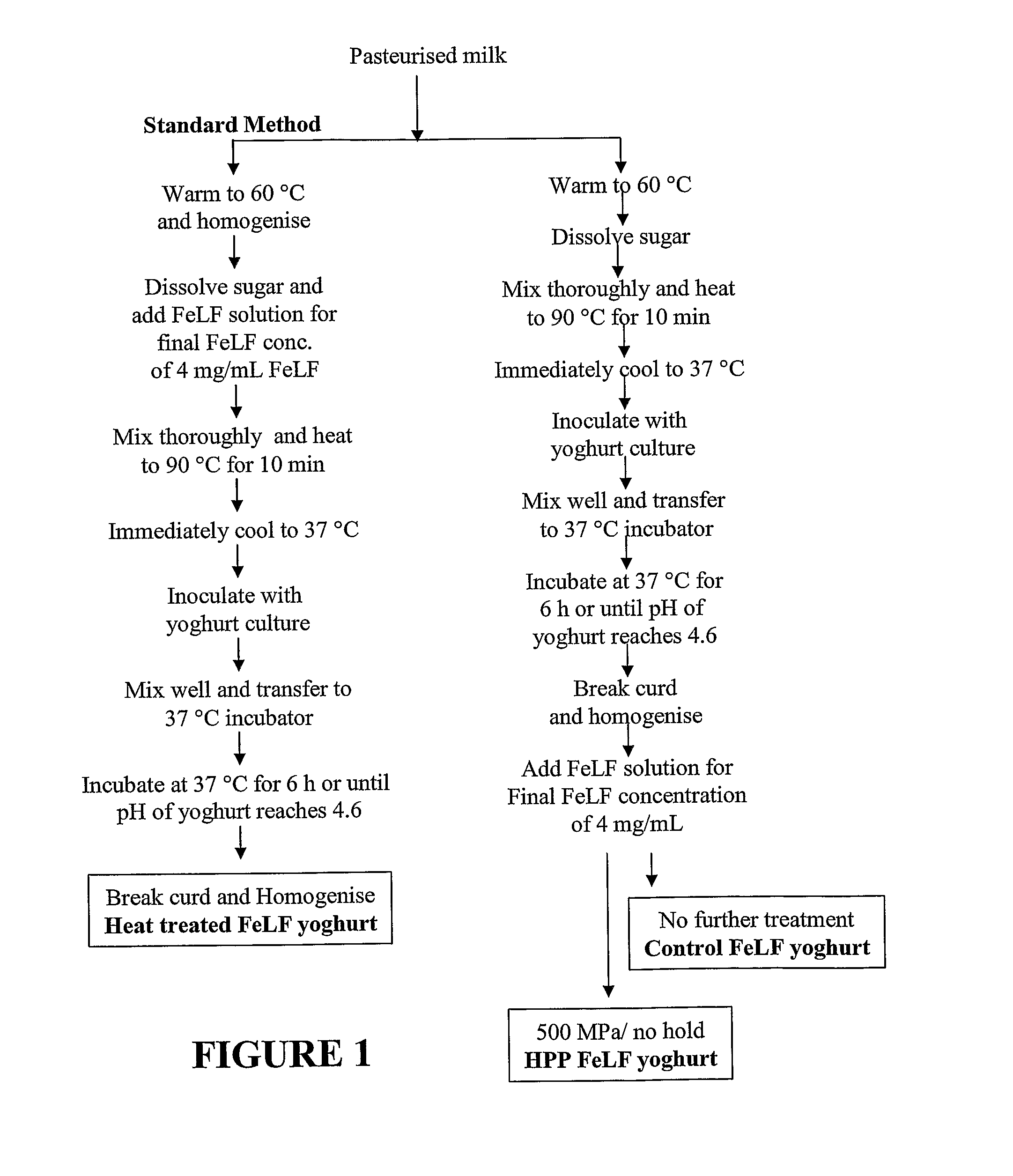
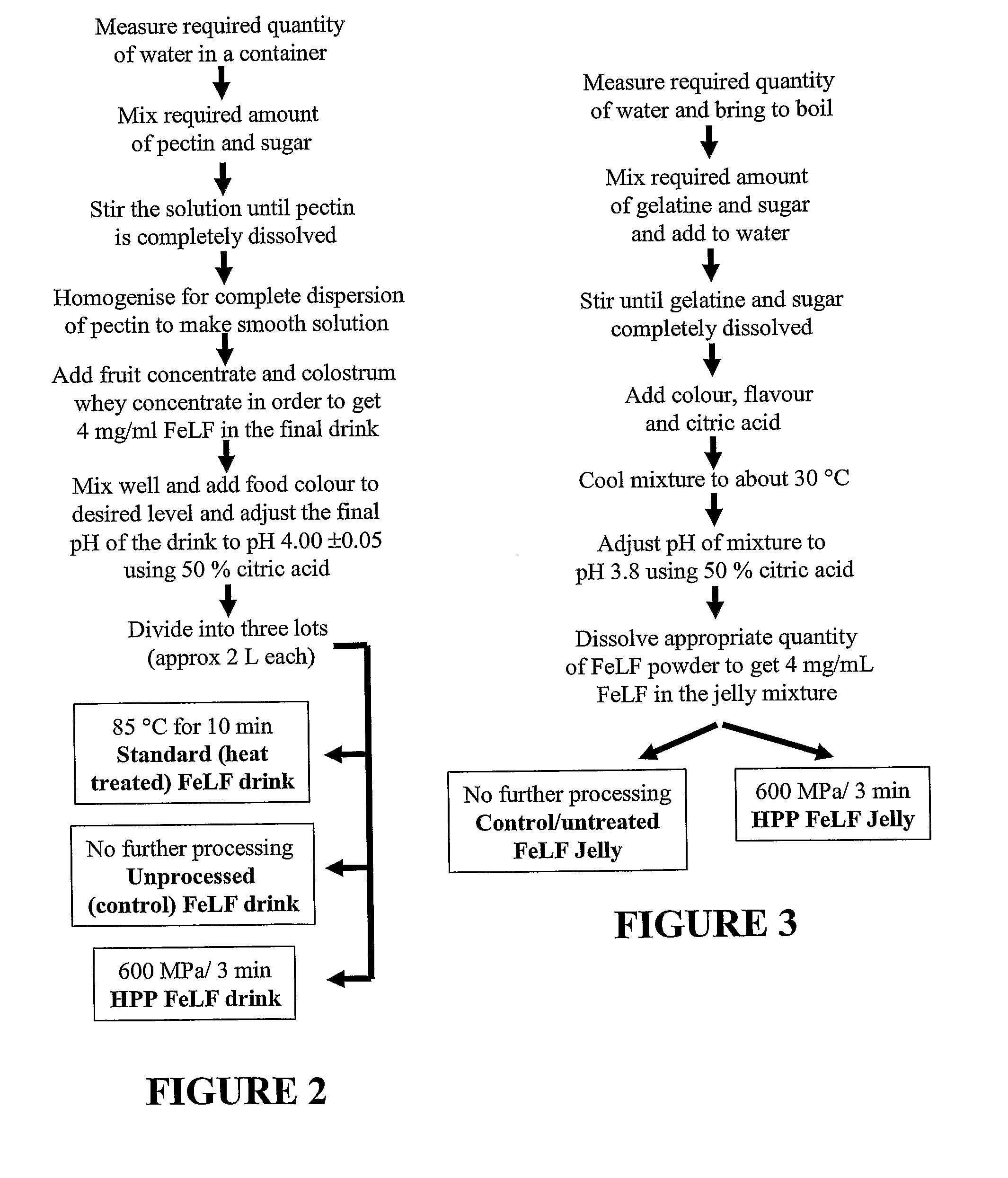
Image
Examples
example 1
[0200]A 6% (w / v) native (˜15% iron saturated) lactoferrin solution prepared as described above was translucent and a burnt orange colour. A solution of apo-lactoferrin (˜0% iron saturated) was clear. Heat treatment of native lactoferrin over the pH range 3.8-7.0 showed that mild conditions (for example, 75° C. for 5 min) induced some colour change in the test solutions indicating loss of iron or oriented iron-binding in lactoferrin, particularly at low pH (Table 1). Higher temperatures (85° C. to 90° C. for 5 min) resulted in virtually complete loss of colour in all solutions over the pH range. At pH 7.0, a thick precipitate formed indicating extensive denaturation of lactoferrin.
TABLE 1Effect of heat treatment on native lactoferrin as a function of pHpH:Treatment Conditions:3.33.84.05.27.0Controlclear++++++++++75° C. / 5 minclearalmost+++++clear85° C. / 5 minclearclearclearclearthick ppt90° C. / 5 minclearclearclearclearthick ppt“+” symbols indicate colour depth on visual inspection. Few...
example 2
[0202]A 6% (w / v) 100% iron saturated lactoferrin solution prepared as described above was translucent and a dark burgundy colour. Heat treatment of fully iron-saturated lactoferrin (˜100%) at pH 3.8, 4.8 and 7.0 (85° C. for 10 min and 90° C. for 10 min) resulted in some loss of colour (at pH 3.8 and 4.8) indicating a loss of bound iron and complete aggregation and loss of soluble lactoferrin in the case of the pH 7.0 sample (data not shown).
[0203]In contrast, pressure treatment at 600 MPa for up to 30 min resulted in no visible colour change for any of the solutions (Table 3).
TABLE 3Effect of pressure and heat treatment on 100% ironsaturated lactoferrin as a function of pHpH:Treatment Conditions:3.84.8Controlburgundyburgundy600 MPa / 5 minburgundyburgundy600 MPa / 30 minburgundyburgundy85° C. / 10 minorange-redorange-red95° C. / 10 minorange-redorange-red
example 3
[0204]Samples of a fully iron-saturated lactoferrin solution (˜100%) were adjusted to pH 7.0, 4.8 or 3.8 and subjected to high pressure at 600 MPa for 5 min or 30 min or heat treatment at 85° C. for 10 min or 95° C. for 10 min. The integrity of lactoferrin, bound iron and iron-binding capacity were determined for each sample. Untreated samples at each pH served as controls. In the case where there were precipitates or insoluble aggregates formed as a result of treatment, the samples were centrifuged at 8000×g for 30 min, at 10° C. and the supernatant taken for analysis.
[0205]Table 4 shows the results from HPLC. Quantitation was by integration of peaks, to give total soluble lactoferrin as a percentage compared to an untreated control. Peak shape was used as a qualitative index of denaturation.
TABLE 4Effect of Heat and Pressure on Iron-saturated Lactoferrinassessed with RP-HPLCpH 3.8pH 4.8pH 7.0Control100100100600 MPa × 5 min9398102600 MPa × 30 min103929285° C. × 10 min92820.390° C. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com