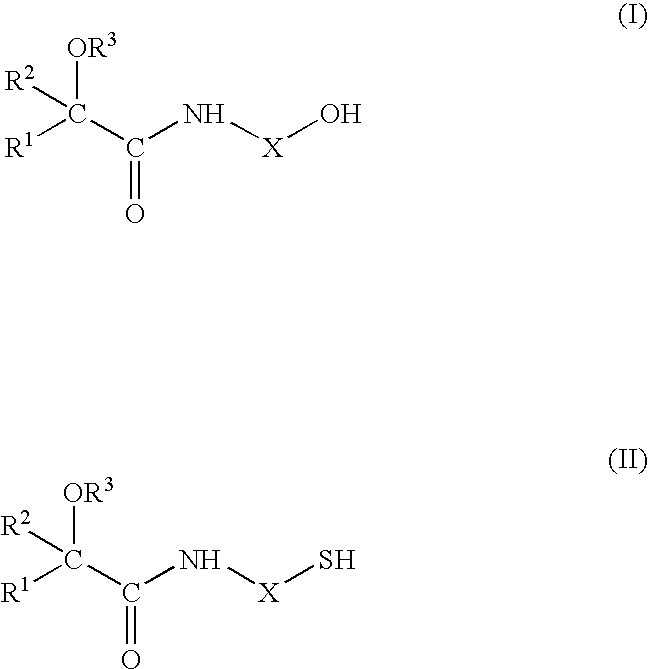
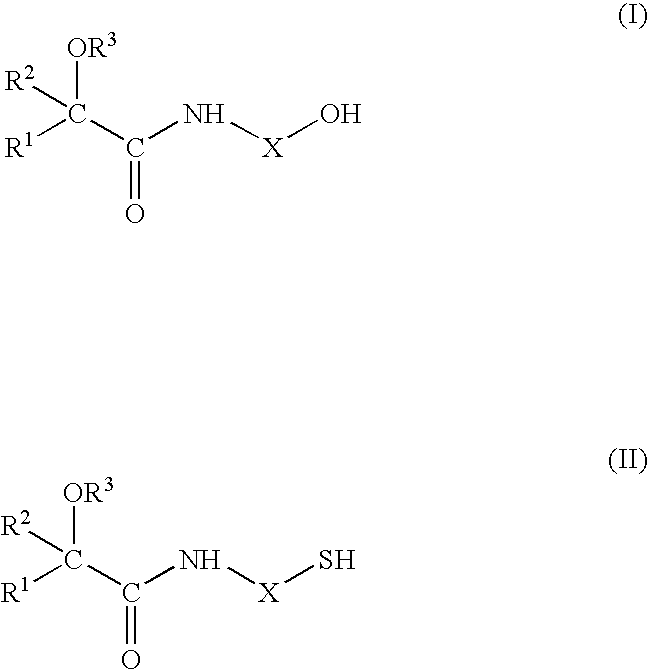
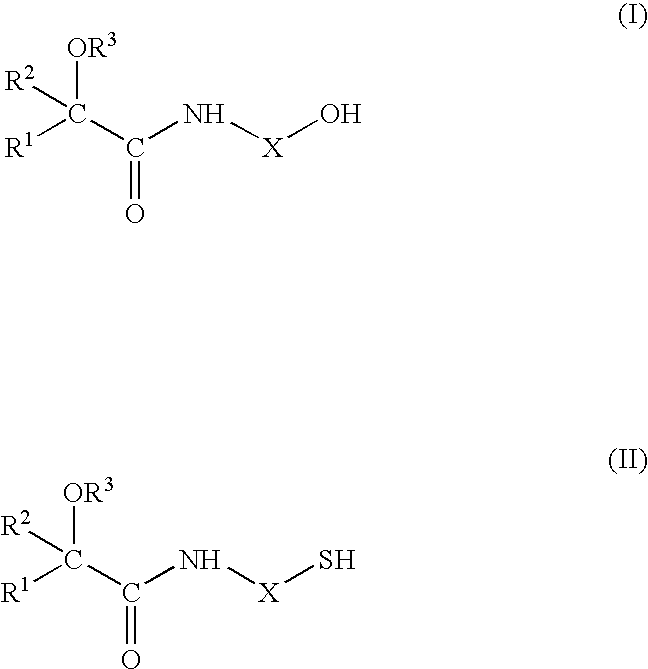
Flavour Compositions
a composition and flavour technology, applied in the field of flavour compositions, can solve the problems of a very complex and important taste system, and achieve the effect of improving the taste of foodstuffs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of N-lactoyl 1-amino-2-methyl-2-propanol:
[0065]a) Preparation of 1-amino-2-methyl-2-propanol from 2,2-dimethyl oxirane and ammonia:
[0066]The reaction was carried out in a closed tube placed in a steam reaction block. The tube was charged with 20 g of a 32% aqueous ammonia solution and 5 g 2,2-dimethyl oxirane and kept at a temperature of 95° C. for 2 hours. Then the tube was cooled to room temperature. The excess ammonia was evaporated by heating the reaction mixture to 70° C. Water was removed on a rotary evaporator. 4.5 g of 1-amino-2-methyl-2-propanol was obtained and analyzed by GC.
b) Preparation of N-lactoyl 1-amino-2-methyl-2-propanol:
[0067]4.2 g crude 1-amino-2-methyl-2-propanol was added to 10.5 g ethyl lactate at room temperature. The mixture was heated to 130° C. and stirred for 3 hours at 125-130° C. The ethanol formed was distilled off during the reaction. The reaction mixture was cooled to room temperature and subsequently transferred to a 50 ml distillation...
example 2
Preparation of N-lactoyl 1-amino-2-propanol:
[0070]22.6 g 1-amino-2-propanol was added to 56.5 g ethyl lactate at room temperature. The mixture was heated to reflux and stirred for 4 hours. The reaction mixture was cooled and left to stand overnight at room temperature. The following day the ethanol formed and the excess of ethyl lactate were removed from the reaction mixture by distillation, using a 20 cm vigreux column. 43 g of N-lactoyl 1-amino-2-propanol (pale brown viscous liquid) was collected as residue and cooled to room temperature. The purity of the obtained product was confirmed by NMR analysis.
example 3
Preparation of N-lactoyl 2-amino-1-propanol:
[0071]3.7 g ethyl lactate and 2.1 g 2-amino-1-propanol were mixed and subsequently heated to 110-115° C. for 2.5 hours. During the reaction, the ethanol formed was distilled off. After the reaction the excess of ethyl lactate was removed by vacuum distillation (bottom temperature: 150° C., 1 mbar), using a 20 cm vigreux column. 3.9 g of residual N-lactoyl 2-amino-1-propanol (brown viscous liquid) was collected, the purity of which was confirmed by NMR analysis. The yield of the reaction was 95%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com