Small Peptides And Methods For Treatment Of Arthritis
a small peptide, arthritis technology, applied in the direction of peptide/protein ingredients, immunological disorders, drug compositions, etc., can solve the problems of affecting the inflammatory response, so as to prevent the involvement of the inflammatory response. , the effect of increasing the inflammatory respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
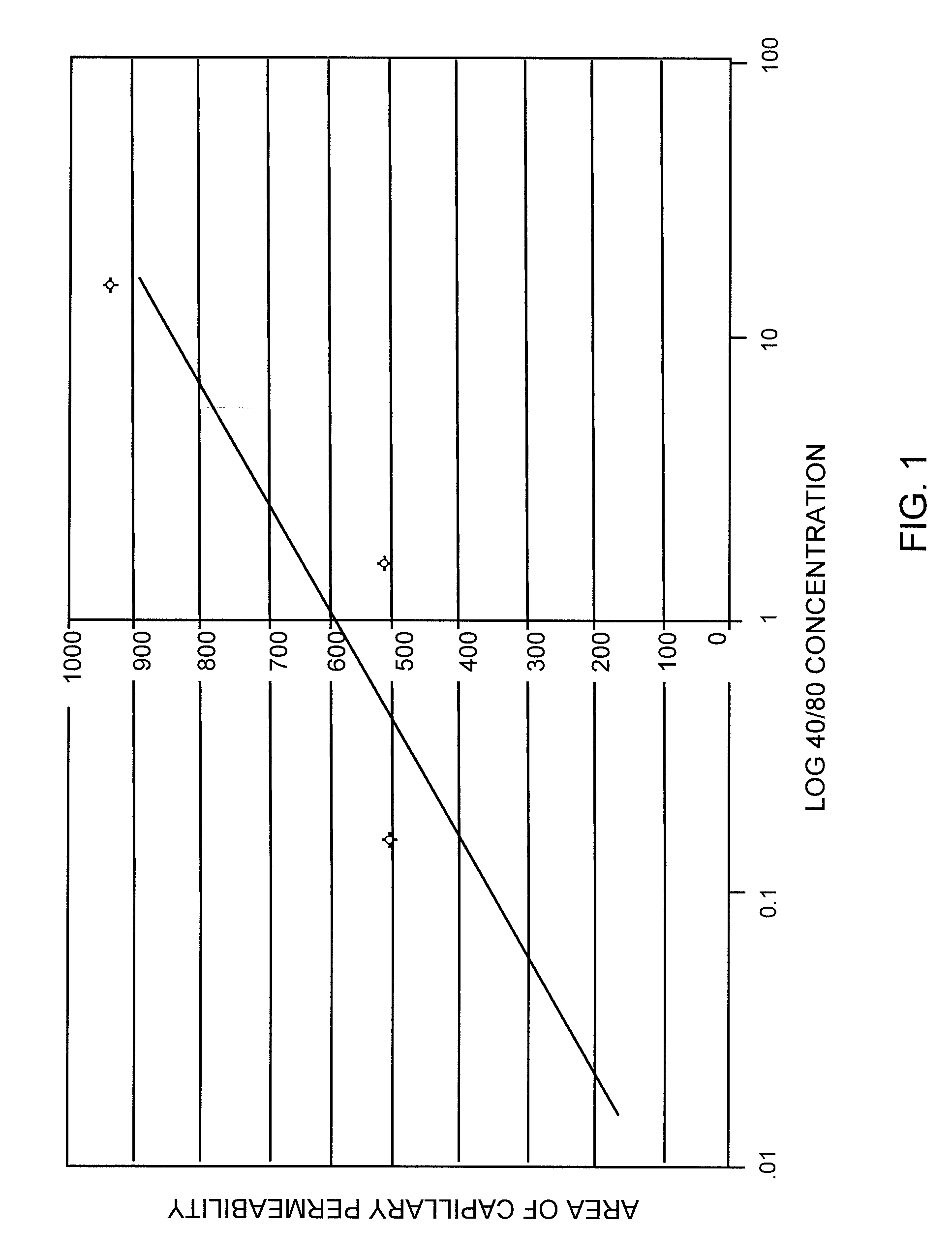
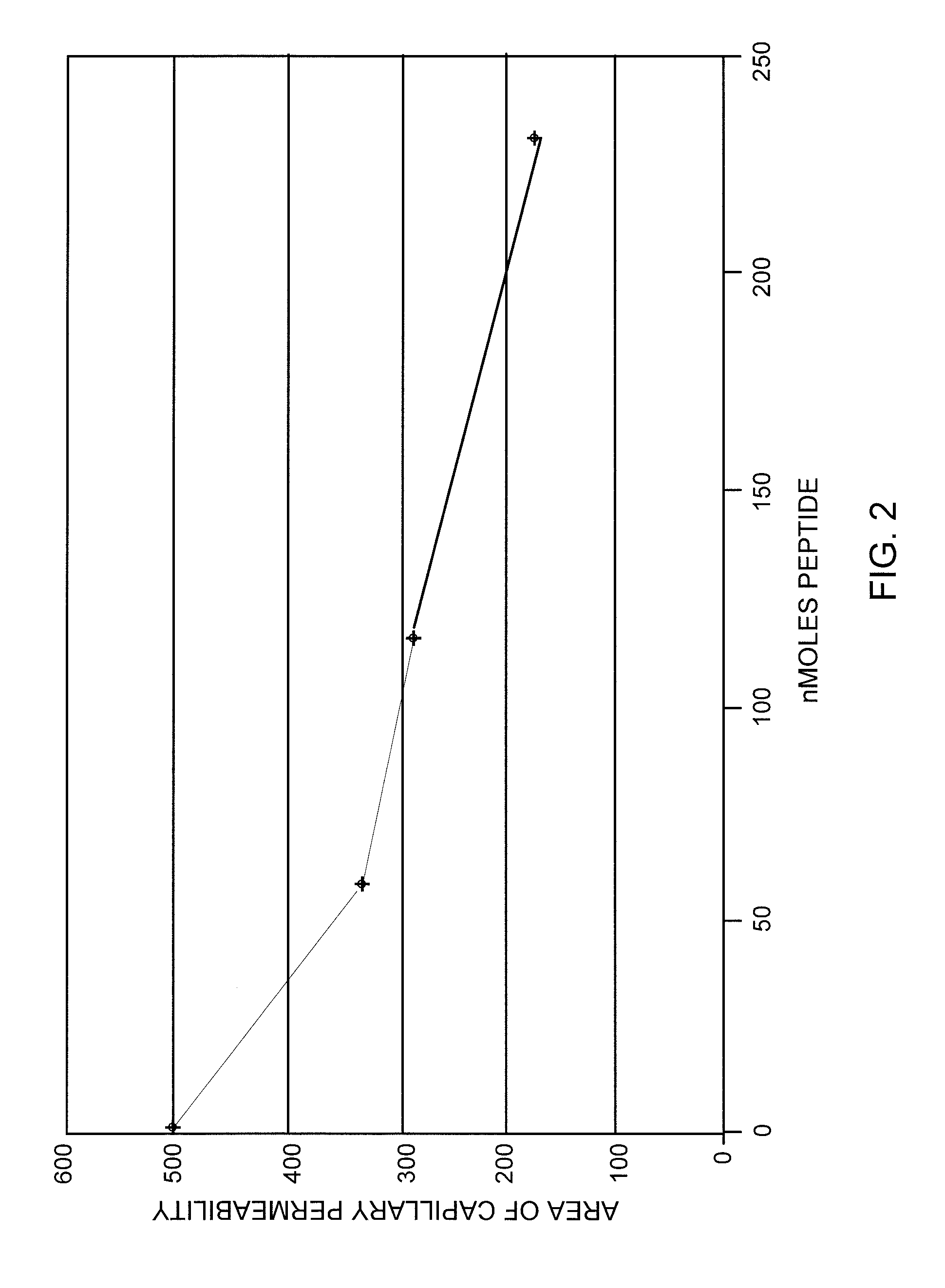
[0070]A dose response curve was prepared for the standard compound, f-Met-Leu-Phe, using the selected dose of 0.15 μg Compound 48 / 80. Doses of 0 to about 230 nM of f-Met-Leu-Phe were tested and the results are shown in FIG. 2. Inhibition of degranulation induced by Compound 48 / 80 was clearly shown.
examples 2-11
[0071]Several f-Met-Leu peptides were tested for inhibition of induced degranulation in the rat skin model using 100 nanomoles of the test peptide and a dose of 0.15 μg Compound 48 / 80. An intrinsic zero-peptide-dose 48 / 80 control was included in each rat for each experiment, and the % of inhibition was expressed in relative terms to this control (0% inhibition). The percent mast cell degranulation produced by 48 / 80 was also determined. The results are tabulated below.
TABLE 1SEQ%% Degran-ExamplePeptideIDInhibitionulation*2f-Met-Leu-Phe (prior art)30603N-acetyl-Met-Leu-Phe0984N-t-BOC-Met-Leu-Phe0—5f-Met-Leu-(iodo)Phe0—6f-Met-Leu-Phe(benzylamide)0—7f-Met-Leu-Phe-Lys40—8f-Met-Leu-Phe(methyl ester)0—9f-Met-Leu-Phe-Phe21001-310f-Met-Leu-Tyr553011f-Met-Leu-Tyr-Tyr50—
example 12
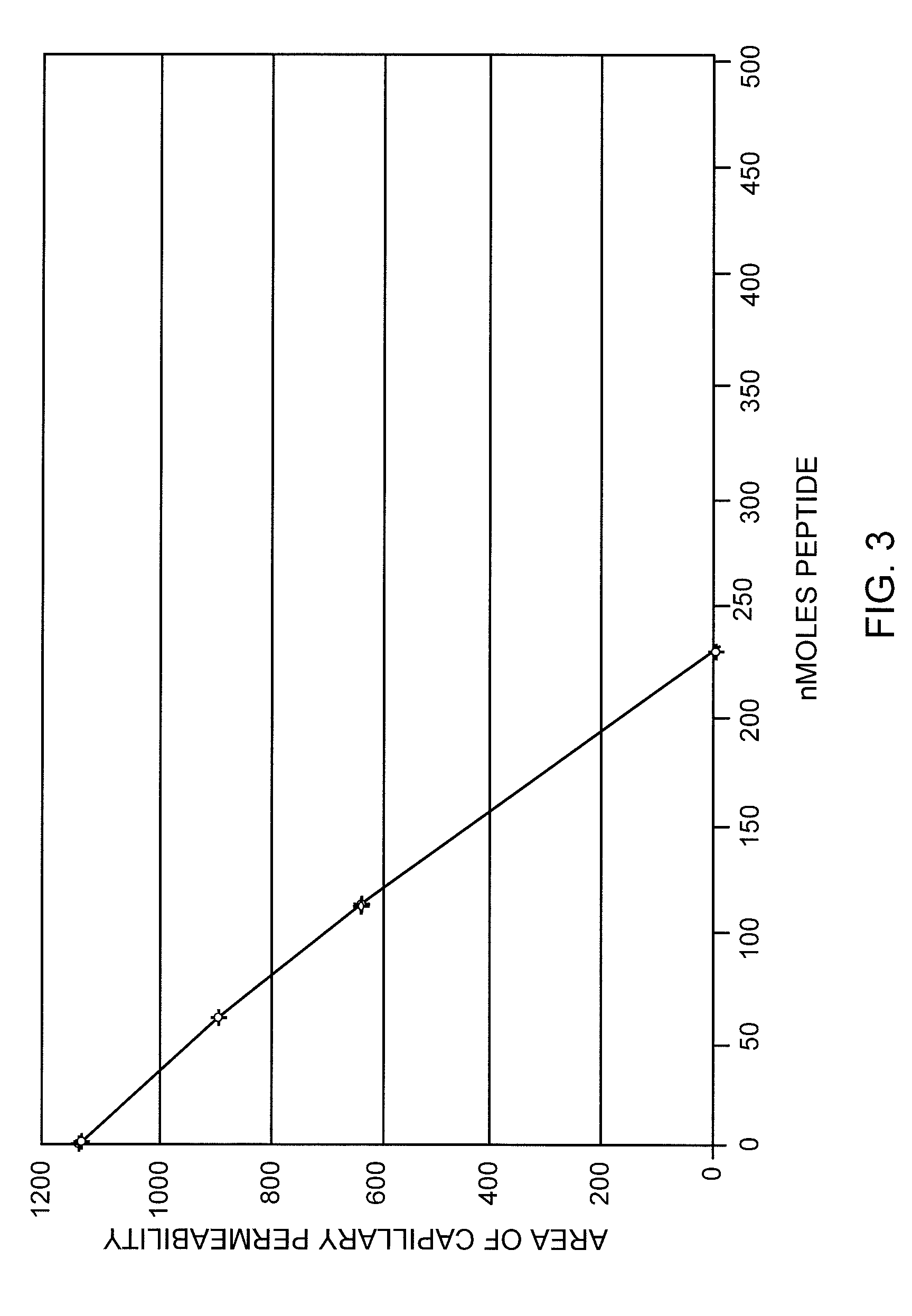
[0072]A dose response curve was prepared for f-Met-Leu-Phe-Phe using the selected dose of 0.15 μg Compound 48 / 80. Doses of 0 to about 230 nM of f-Met-Leu-Phe-Phe were tested and the results are shown in FIG. 3. Surprisingly remarkable inhibition of degranulation induced by Compound 48 / 80 was clearly shown. The inhibition of induced degranulation for f-Met-Leu-Phe-Phe was unexpectedly substantially better than that of the standard compound f-Met-Leu-Phe.
The Ova-Induced Bronchial Asthma Mouse Model for Inhibition of Mast Cell Degranulation
[0073]Asthma is a complex disease, which is characterized by spontaneous exacerbation of airways obstruction and persistent bronchial hyperresponsiveness. Chronic infiltration with activated T-lymphocytes, eosinophils and macrophages / monocytes of the airway submucosa is another established feature. Inflammatory mechanisms, with expression of cytokines, and the release of inflammatory mediators, underlie the pathogenesis of bronchoconstriction and bro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com