Chemical probe compounds that become fluorescent upon reduction, and methods for their use
a technology of chemical probe compounds and fluorescent compounds, applied in the field of quenching chemical stain compounds, to achieve the effect of reducing the quenching ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of compound (5)
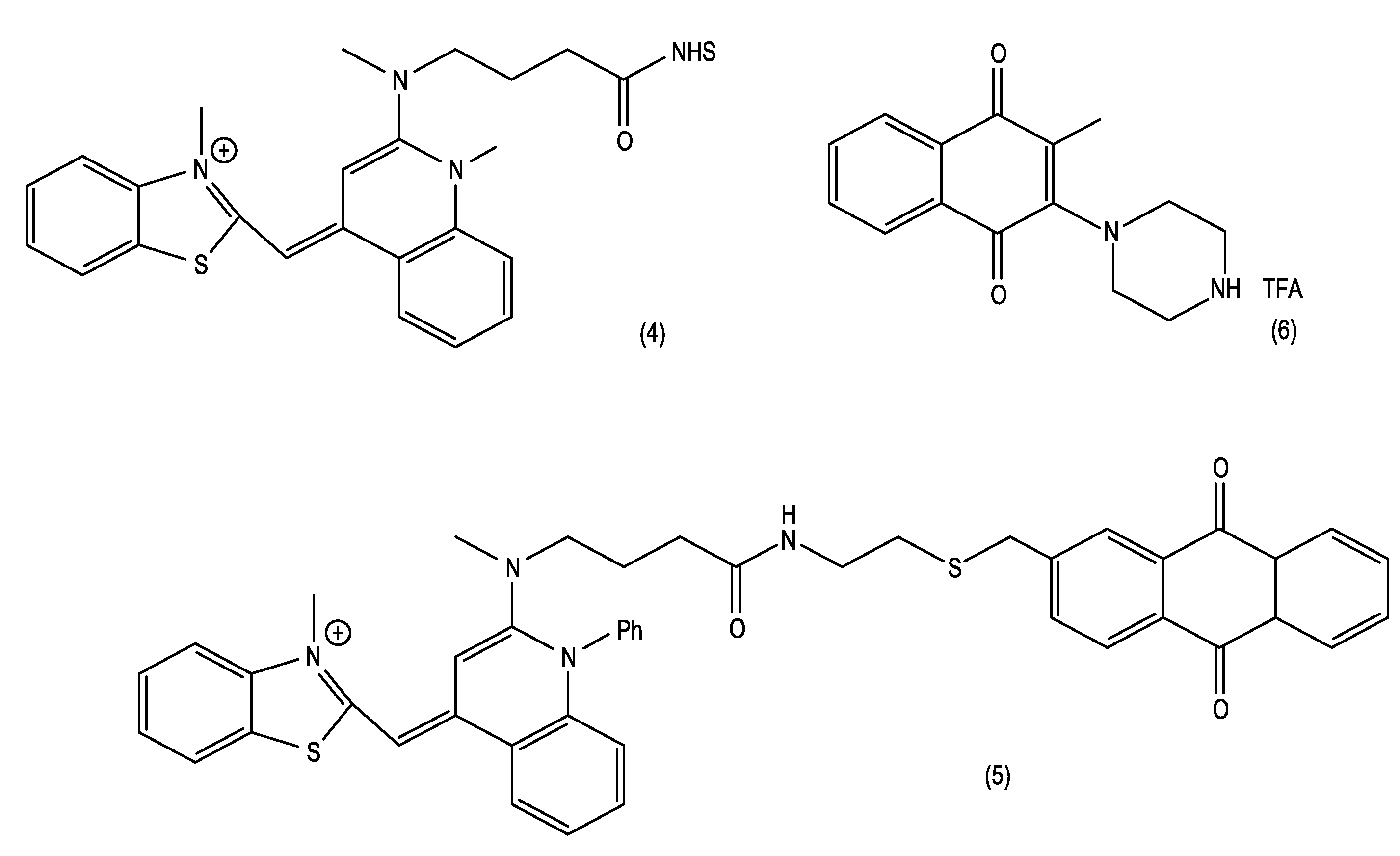
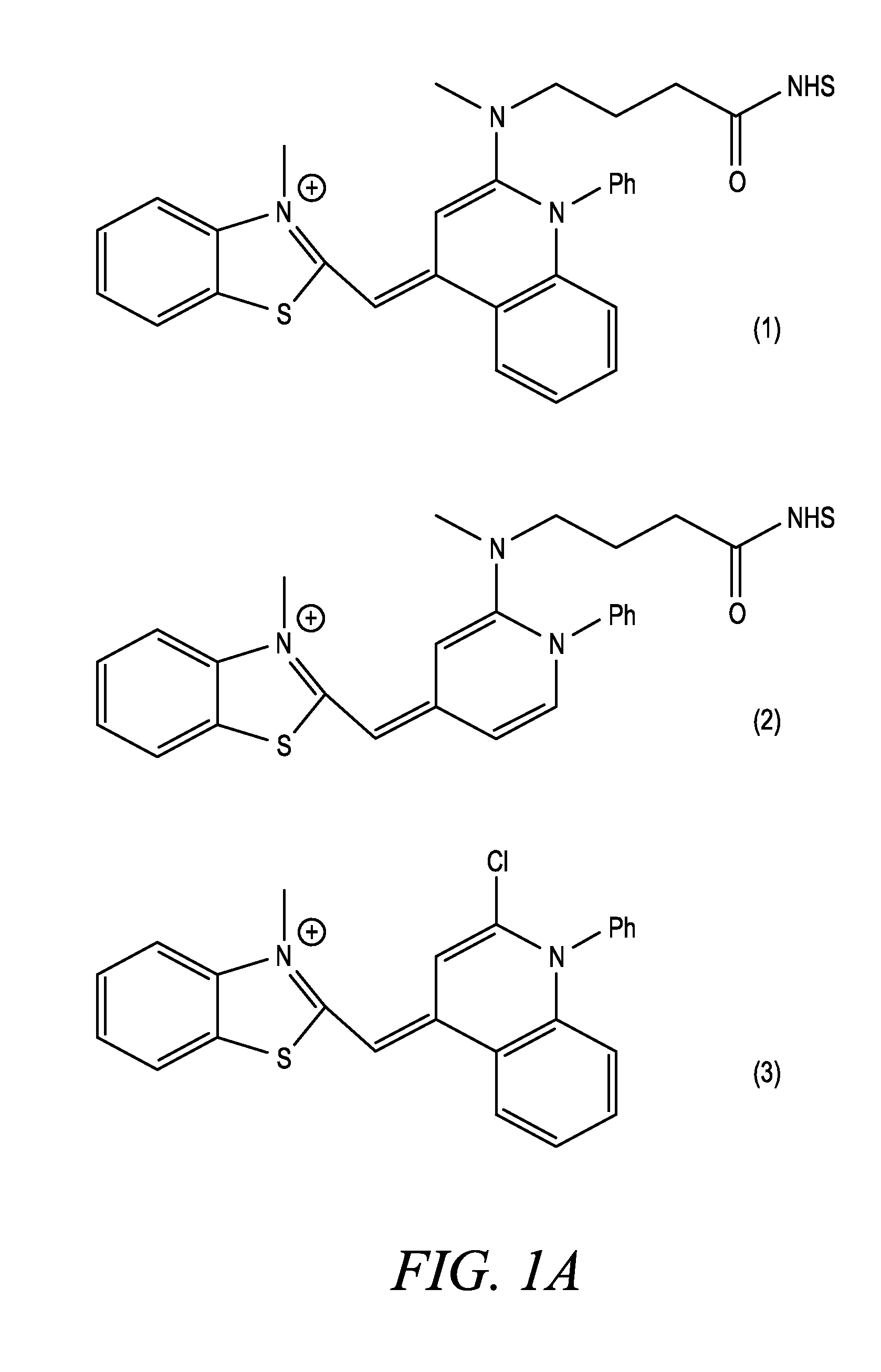
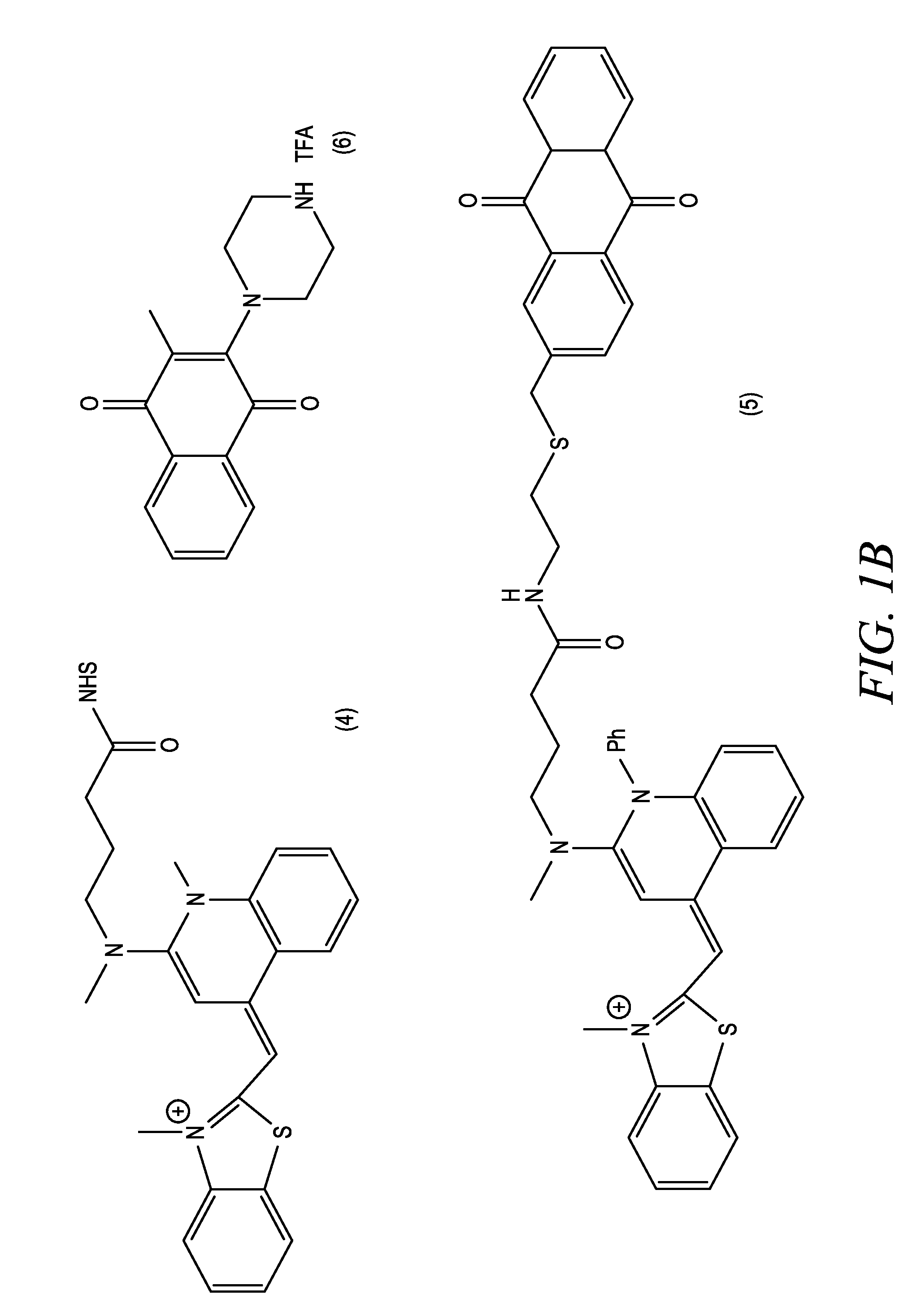
[0045]A mixture of 40 mg compound (1), 60 mg 2-(2-aminoethylthio)methylanthraquinone, and 45 microliters triethylamine was stirred in 1 mL dimethylformamide overnight at room temperature. Product compound (5) was purified by column chromatography on silica gel with 4:4:3:1 ethyl acetate:chloroform:methanol:acetic acid.
example 2
Preparation of Compound (6)
[0046]A mixture of 4.4 g of 2-methyl-1,4-napthoquinone and 11.9 g of 1-tBoc-piperazine was heated in 90 mL of a methanol / dichloromethane (1:1, v / v) solvent mixture at about 45° C. for 24 hours. The solvent was evaporated and the crude product was purified on a silica gel column to yield 1.08 g of desired intermediate. Trifluoroacetic acid (1 mL) was added to 100 mg of this tBoc-protected piperazine in 5 mL of dichloromethane, and after one hour at room temperature, all of the volatile components were removed and the product compound (6) was used “as is” without further purification.
example 3
Preparation of Compound (7)
[0047]Triethylamine (0.21 mL) was added to a mixture of 70 mg of compound (3) and 0.28 mmole of compound (6) in 5 mL of dichloroethane, and the resulting reaction mixture was heated at 55-60° C. for one hour. The reaction was cooled to room temperature and volatile components were evaporated. The crude product compound (7) was purified using silica gel column chromatography with 2:2:1 ethyl acetate:chloroform:methanol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| structure | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
| energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com