Isolation and growth of stem cells from hemangiomas
a technology of stem cells and hemangiomas, which is applied in the field of stem cells, can solve the problems of creating cosmetic problems, not always cosmetically acceptable, and each conventional treatment option carries potential side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
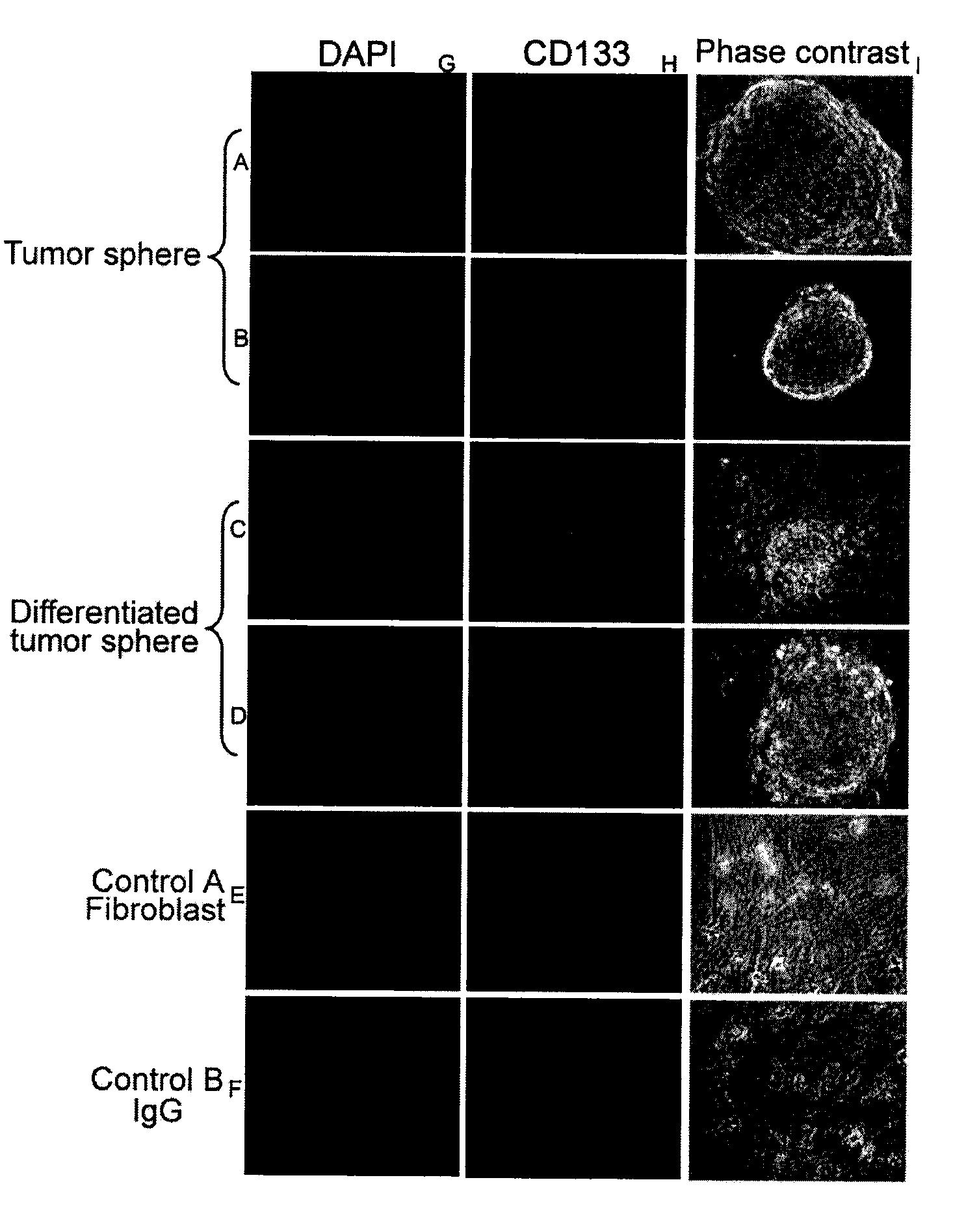
Isolation of Tumor Spheres
[0161]Tissue samples were isolated from superficial raised patches on the skin of male and female infants (less than 1 year), prior to involution. Upon receipt, the tissue was minced and digested with collagenase for 2 hours at 37° C. Following digestion and several wash steps, the tissue mixture was then filtered through a 100 μm cell strainer and grown in culture using KNOCKOUT™ Dulbecco's Modified Eagle's Medium (DMEM), 10% KNOCKOUT™ Serum, 1× non-essential amino acids (NEAA), and 20 μg / ml basic-fibroblast growth factor (bFGF). Following growth of this primary culture for approximately three days, tumor spheres will spontaneously form from the homogenous mixture of primary culture. These spheres are collected using collagenase digestion, washed, and plated at a density of 200 cells per milliliter with DMEM-F12 nutrient mix, 20 μg / ml bFGF and NEAA on low-attachment plates. The tumor spheres form and propagate using collagenase digestion, which digest the ...
example 2
[0167]Isolated cells from tumor spheres (1×106 cells / well in 6-well plates), are processed using a ChIP Assay Kit (Upstate, Charlottesville, Va.) following the manufacture's protocol. Briefly, cells are cross-linked by adding formaldehyde (27 μl of 37% formaldehyde / ml) and incubated for 10 min. Then, chromatin is sonicated to an average size of approximately 500 bp and immunoprecipitated with SALL4 antibodies, preimmune serum, or anti-HA (hemagglutination) antibody. Antibodies for histone modifications, histone H3 trimethy K4 and histone H3 dimethy K79, may be purchased from Abeam (Cambridge, Mass.). Histone-DNA crosslinks are reversed by heating at 65° C. followed by digestion with proteinase K (Invitrogen, Carlsbad, Calif.). DNA is recovered by using a PCR purification kit (Qiagen, Valencia, Calif.) and then used for PCR or QRT-PCR (quantitative real time polymerase chain reaction).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Cell proliferation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com