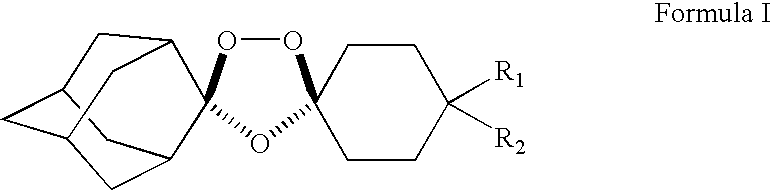
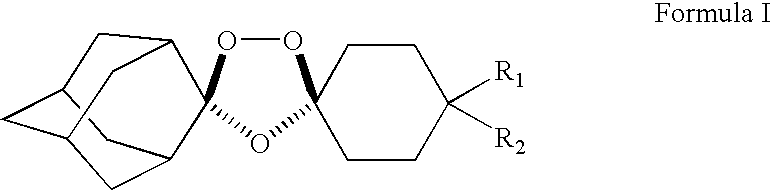
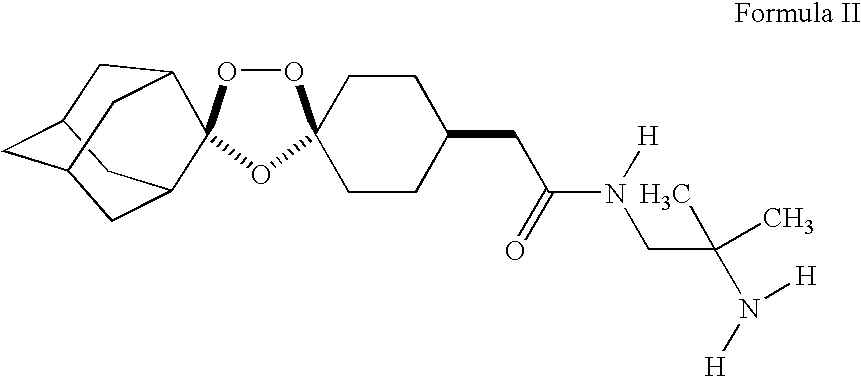
Stable dosage forms of spiro and dispiro 1,2,4-trioxolane antimalarials
a technology of trioxolane and spiro, which is applied in the direction of antiparasitic agents, drug compositions, active ingredients of phosphorous compounds, etc., can solve the problems of malaria, the most common parasitic disease of humans, and the characteristic paroxysm of the disease, and achieves the effect of death, reducing the risk of infection, and improving the effect of anti-malarial
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0066]
Ingredients% w / wIntragranularMaleate salt of a compound of Formula II (active43.2compound)Microcrystalline Cellulose46.67Magnesium stearate0.75ExtragranularMicrocrystalline Cellulose5.63Croscarmellose sodium3.0Magnesium stearate0.75CoatingOpadry ® OY SS 58910 white2.5Waterq.sTotal weight615Water content
Procedure:
[0067]1. Active compound and intragranular portion of microcrystalline cellulose were sieved through sieve BSS# 44 and mixed together in a double cone blender to form a uniform blend.
[0068]2. To the blend of step 1, intragranular portion of sifted magnesium stearate was added and blended for about 5 minutes.
[0069]3. The blend of step 2 was compacted in a roller compactor and was sifted through sieve BSS # 22 to form granules.
[0070]4. Extragranular portion of microcrystalline cellulose, croscarmellose sodium and magnesium stearate were sieved through sieve BSS # 44 and blended with the granules of step 3.
[0071]5. The blend of step 4 was compressed using suitable size pu...
example 2
[0075]
Ingredients% w / w1Maleate salt of a44.33compound of Formula II(active compound)2Microcrystalline Cellulose51.173Magnesium stearate1.54Croscarmellose sodium3.0Total weight600 mgWater content
Procedure:
[0076]1. Active compound, microcrystalline cellulose, croscarmellose sodium and magnesium stearate were sifted through sieve BSS# 44.
[0077]2. Sifted active compound, microcrystalline cellulose, and croscarmellose sodium were mixed in a double cone blender for about 15 minutes to form a uniform blend.
[0078]3. To the blend of step 2, sifted magnesium stearate was added and mixed for about 5 minutes.
[0079]4. The blend obtained in step 3 was directly compressed using suitable size capsule shape punches to obtain compressed tablets.
examples 3 and 4
[0080]
IngredientsExample 3 % w / wExample 4 % w / wIntragranularMaleate salt of a compound of7.6813.8Formula II (active compound)Piperquine phosphate61.8055.5Microcrystalline Cellulose20.3921.05Magnesium stearate0.440.39Crospovidone2.211.99ExtragranularMicrocrystalline Cellulose4.423.99Crospovidone2.211.99Magnesium stearate1.051.09CoatingOpadry ® O2B53782 orange2.52.5Waterq.sq.sTotal weight (mg)1332.5738Water content
Procedure:
[0081]1. Active compound, piperaquine phosphate and intragranular portion of microcrystalline cellulose and crospovidone were sieved through sieve BSS # 44 and mixed together in a double cone blender to form a uniform blend.
[0082]2. To the blend of step 1, intragranular portion of sifted magnesium stearate was added and blended for about 5 minutes.
[0083]3. The blend of step 2 was compacted in a roller compactor and was sifted through sieve BSS # 18 to form granules.
[0084]4. Extragranular portion of microcrystalline cellulose and crospovidone were sieved through sie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com