Methods for Detecting Papillomavirus DNA in Blood Plasma and Serum
a technology of papillomavirus and blood plasma, which is applied in the direction of peptide sources, p53 proteins, peptides, etc., can solve the problems that hpv infection cannot be detected in all cases of cervical dysplasia, pap smear does not detect all instances of cervical dysplasia, and may be insufficient for interpretation, so as to achieve the effect of assessing the prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
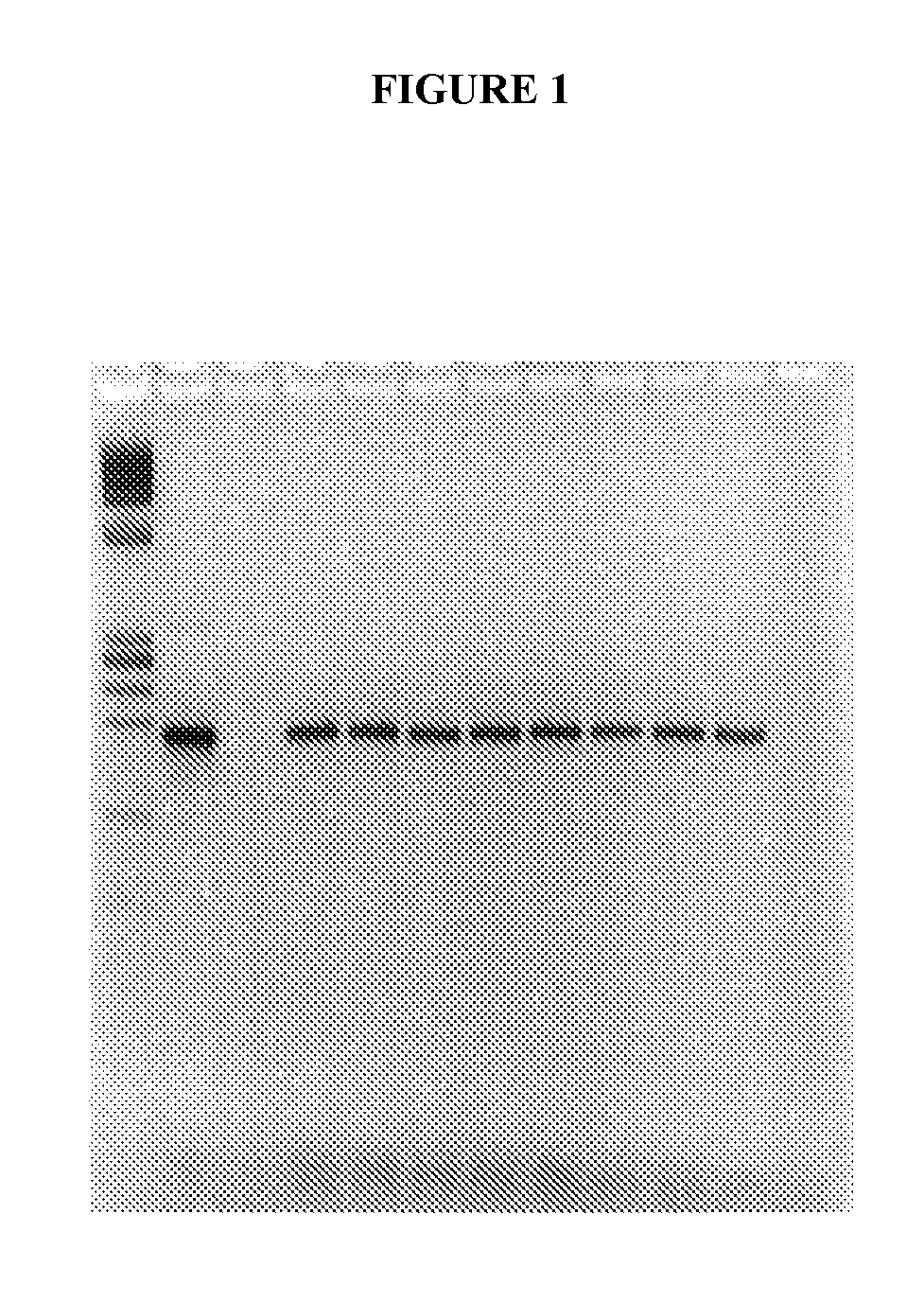
[0046]Three cottontail rabbits were infected at time zero with the cottontail rabbit papilloma virus (CRPV), a form of the virus specific to the squamous epithelium of the cottontail rabbit. The cottontail rabbit is used extensively as a model for the human version of papilloma virus infection and progression to dysplasia and carcinoma. Blood was drawn and serum separated prior to infection and approximately every month thereafter. The serum was stored frozen at −80° C. Each of the animals developed pre-malignant lesions (papillomas) approximately 5 weeks after the initial inoculation, and cancer approximately 4-13 months after time zero.
[0047]DNA was extracted from the serum by a method described in U.S. patent application Ser. No. 08 / 818,058. One tenth of the resulting extract was utilized in a polymerase chain reaction, also as described except that primers to the E8 region of the CRPV genome were used at 25 picomoles each per reaction. The oligonucleotide primer sequences were a...
example 2
[0050]A young, sexually active woman visits her gynecologist for a routine examination. A Pap smear is obtained as standard practice, and blood for the HPV nucleic acid assay is also obtained. The Pap smear is reviewed by a cytotechnologist, who finds no abnormalities. The blood test is performed by isolating nucleic acids, both DNA and RNA, from the plasma or serum component. A broad spectrum assay for several dozen HPV types is performed by the PCR method, with detection by the hybrid capture method commonly used for testing of tissue samples for HPV. A positive result is obtained, and a portion of the PCR product is genotyped by digestion with a number of restriction endonucleases. The patient is found to have two types of virus, high risk type 18 and low risk type 4. The sample is used to quantify the mRNA for these two types and a value of 500 genomes / microliter of blood is obtained for the type 18.
[0051]In retrospect, the “normal” Pap smear may be attributable to lab error, sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermostable | aaaaa | aaaaa |
| nucleic acid amplification | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com