High-sensitivity proteolysis assay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
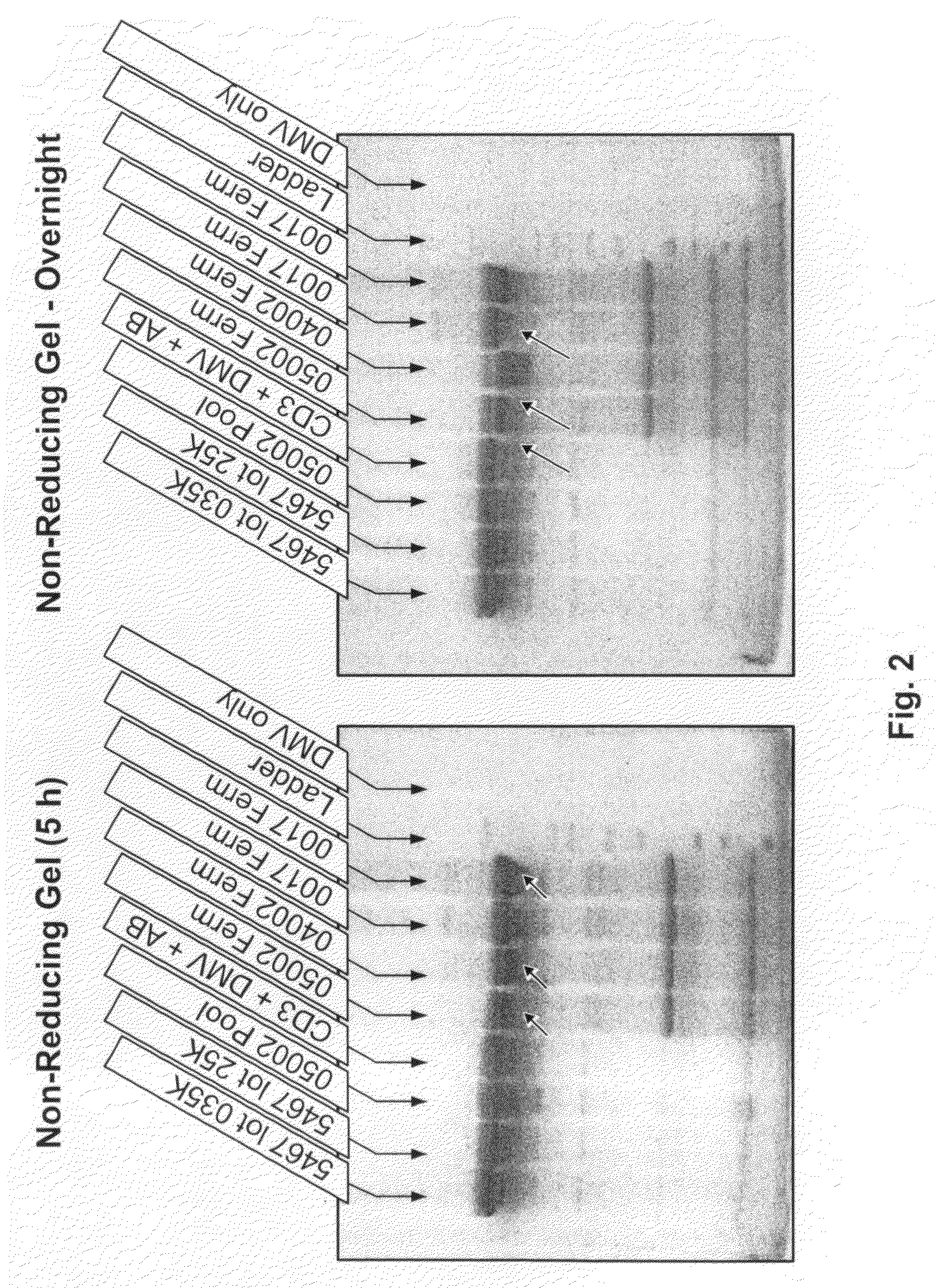
[0057]In this example, the proteolytic activity of a sample of growth media was assayed using an anti-IL-10 or an anti-IGF1R monoclonal antibody as a polypeptide substrate. The growth media used was a chemically defined media into which a hydrolysate was added. The hydrolysate was the source of the proteolytic activity that was determined.
[0058]This example demonstrates that while conventional proteolytic analysis techniques are not sufficient for detecting the low levels of proteolytic activity present in the hydrolysate used, the assays of the present invention are sufficiently sensitive and, thus, well suited for this purpose.
[0059]SDS-PAGE gel. Gels containing 4-12% polyacryamide (stacking gel-resolving gel) were used for non-reducing SDS-PAGE, and gels containing 4-20% polyacryamide (stacking gel-resolving gel) were used for reducing SDS-PAGE.
[0060]1.5M Tris pH 8.8: Dissolved 90.73 g Tris base in 400 ml DI H2O. Adjusted pH to 8.8 with hydrochloric acid. Brought...
example 2
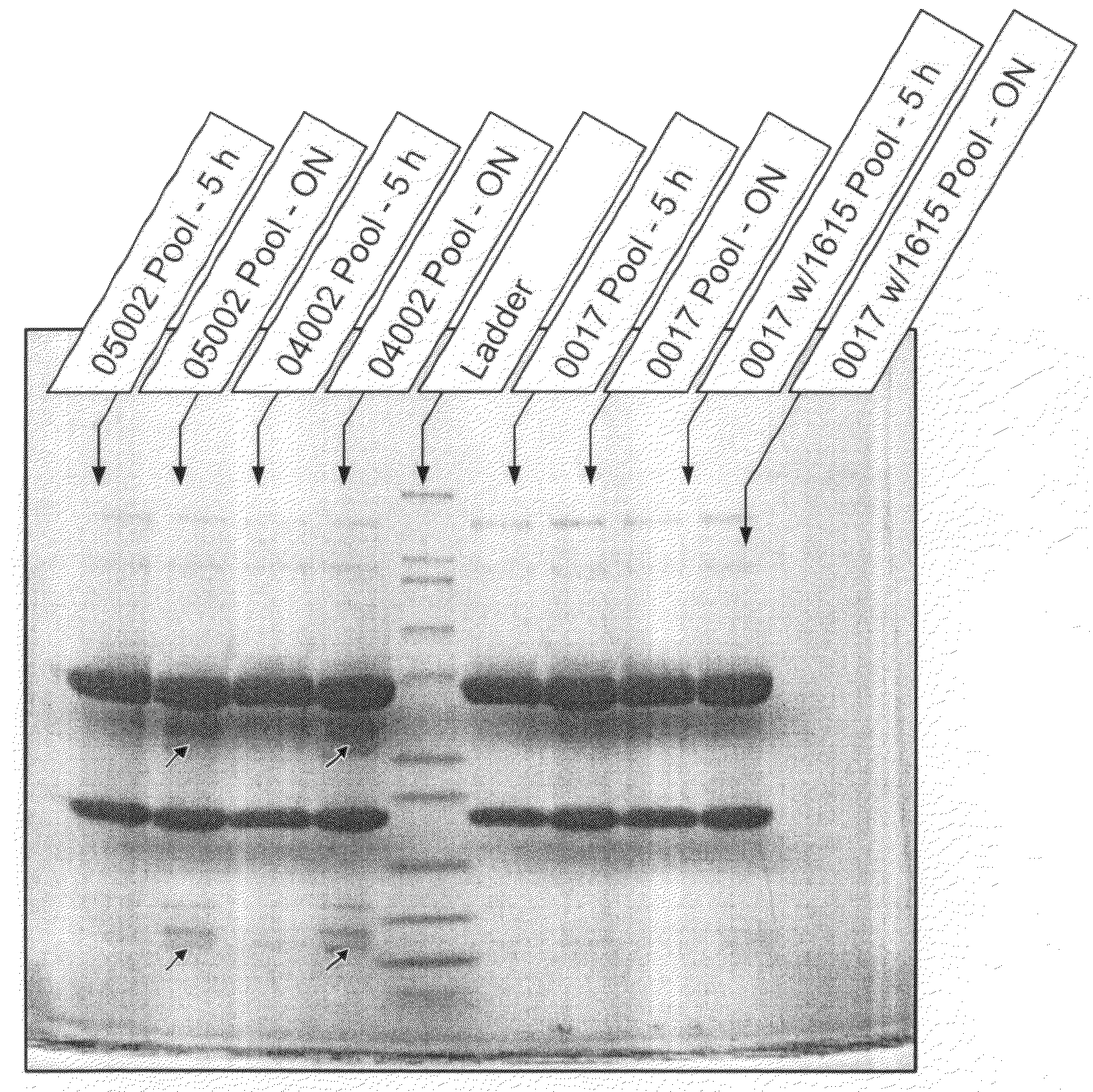
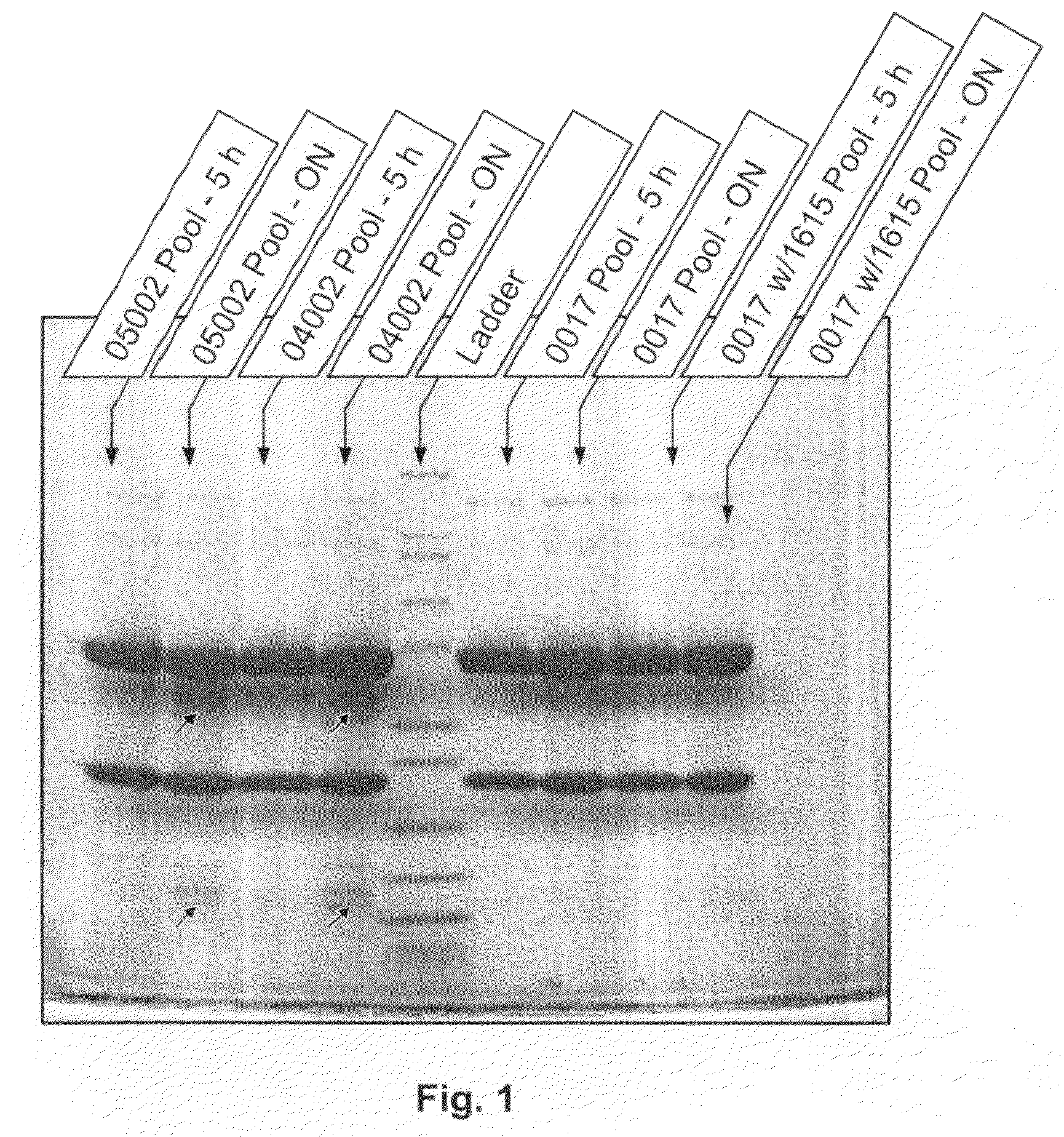
Analysis of Degradation of Anti-IGF1R by Growth Medium
[0068]In this example, the present protease assay was used to evaluate several culture components for proteolytic activity against anti-IGF1R antibody. Using inhibition experiments, it also demonstrates that the fragments detected are indeed from enzymes present in solution instead of being artifacts from the assay itself, as previously seen at pH 6.8.
[0069]Animal-component free C5467 CHO medium, animal-component free imMEDIAte Advantage™ CHO medium (without aurintricarboxylic acid (ATA) or hydrolysates) and 1615 CHO medium supplement (Sterile filtered feed concentration containing amino acids, vitamins, recombinant human insulin, plant hydrolysates, trace elements and other organic compounds; lacking glucose, L-glutamine, phenol red, antibiotics, antimycotics, or transferrin hypoxanthine and thymidine) were obtained from Sigma-Aldrich (St. Louis, Mo.). Hypep 4601S wheat hydrolysates were obtained from Kerry Biosciences (AH Almer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com