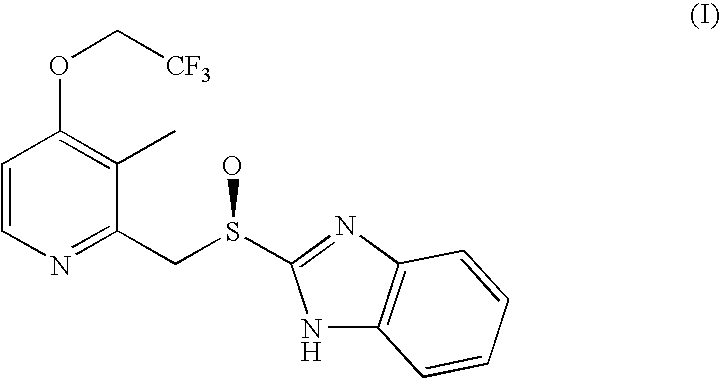
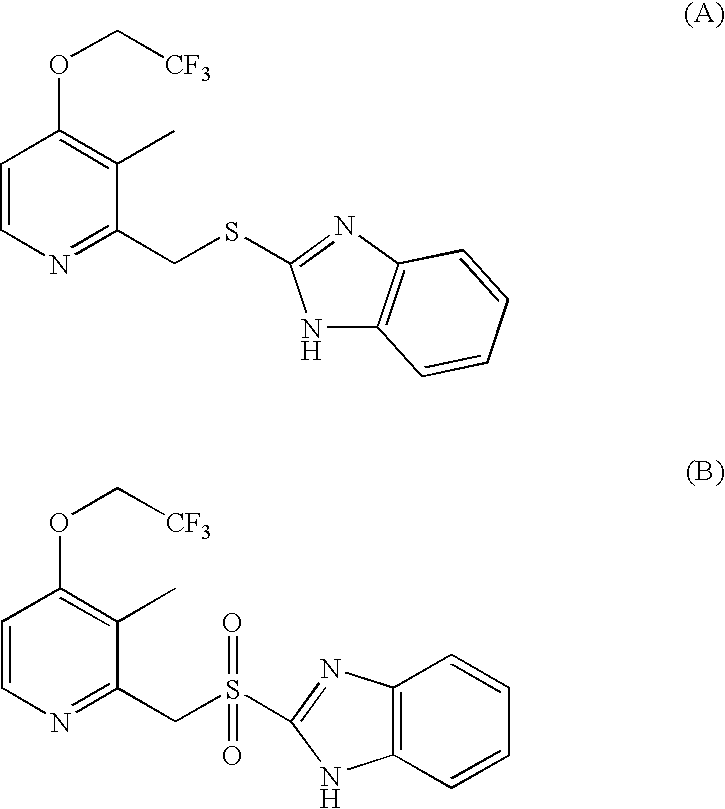
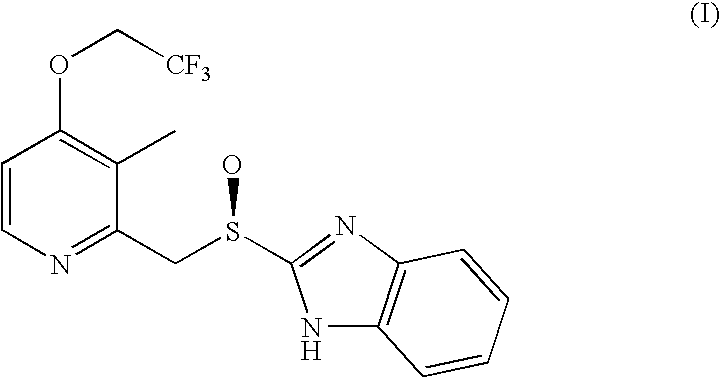
Process for the preparation of dexlansoprazole
a technology of dexlansoprazole and process, which is applied in the field of process for the preparation of dexlansoprazole, can solve the problems of removal of compound (b), complicated purification of this mixture, etc., and achieve the effects of high chemical purity, complete stereoselectivity, and a large mark
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of the (R)-2-[[[3-methyl-4-nitro-2-piridyl]-methyl]sulfinyl]benzimidazole (II)
[0037]A mixture of 2-[[3-methyl-4-nitro-2-piridyl]methylthio]benzimidazole (10 g, 33 mmoles, containing 70 mg of water) and (+)-diethyl-L-tartrate (3.02 g, 14.6 mmoles), in THF (100 ml) is brought to reflux temperature and maintained under stirring for 30 minutes. Titanium isopropoxide (1.89 g, 6.66 mmoles) is added and the mixture is maintained under stirring at reflux temperature till the formation of a clear solution is achieved. The solution is then cooled and added with diisopropylethylamine (1.42 g, 10.9 mmoles). After having achieved the range temperature between −5° C. and 0° C., the solution is treated by slow cooling with cumene hydroperoxide 88% (17.3 g, 100 mmoles). The reaction mixture is maintained under stirring for 3 hours at about 0° C., then treated with a 30% sodium thiosulphate solution to decompose the residue of cumene hydroperoxide. After the separation of the phases the aq...
example 2
Synthesis of Dexlansoprazole (I)
[0038]A solution of a compound of formula (II) (10 g, 31.5 mmoles), obtained by Example 1, in 2,2,2-trifluoroethyl alcohol (50 ml) is treated with Pd(PPh3)4 (36 mg, 0.031 mmoles) and potassium tert-butoxide (17.6 g, 157 mmoles). The so formed solution is maintained under stirring at a temperature of 80° C. for 4 hours and then cooled at room temperature. The reaction mixture is then quenched in water, extracted with methylethylketone. The phases are separated and the organic one is first diluted with water and then brought to pH 9 by adding sodium bisulfite. The phases are separated and the organic one is concentrated under reduced pressure. The residue is dissolved in acetone and then crystallized by adding water slowly to the solution. The obtained crystals are filtered, washed with acetone and copious water. After drying about 11 g of crystalline sesquihydrate Dexlansoprazole (yield 90%), with a purity higher than 99% and an enantiomeric excess hig...
example 3
Synthesis of Dexlansoprazole (I)
[0039]A solution of compound (II) (1.42 Kg) in 2,2,2-trifluoroethanol (6.40 L) is treated with potassium hydroxide (1.31 Kg). The reaction mixture is left to react at 90° C. for 1.5 hours and then cooled to 25° C. Water and toluene are added to the reaction mixture and the two newly formed phases are separated. Pure crystalline Dexlansoprazole sesquihydrate (1.56 Kg, 90% yield) can be isolated from the organic phase following the procedure reported in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com