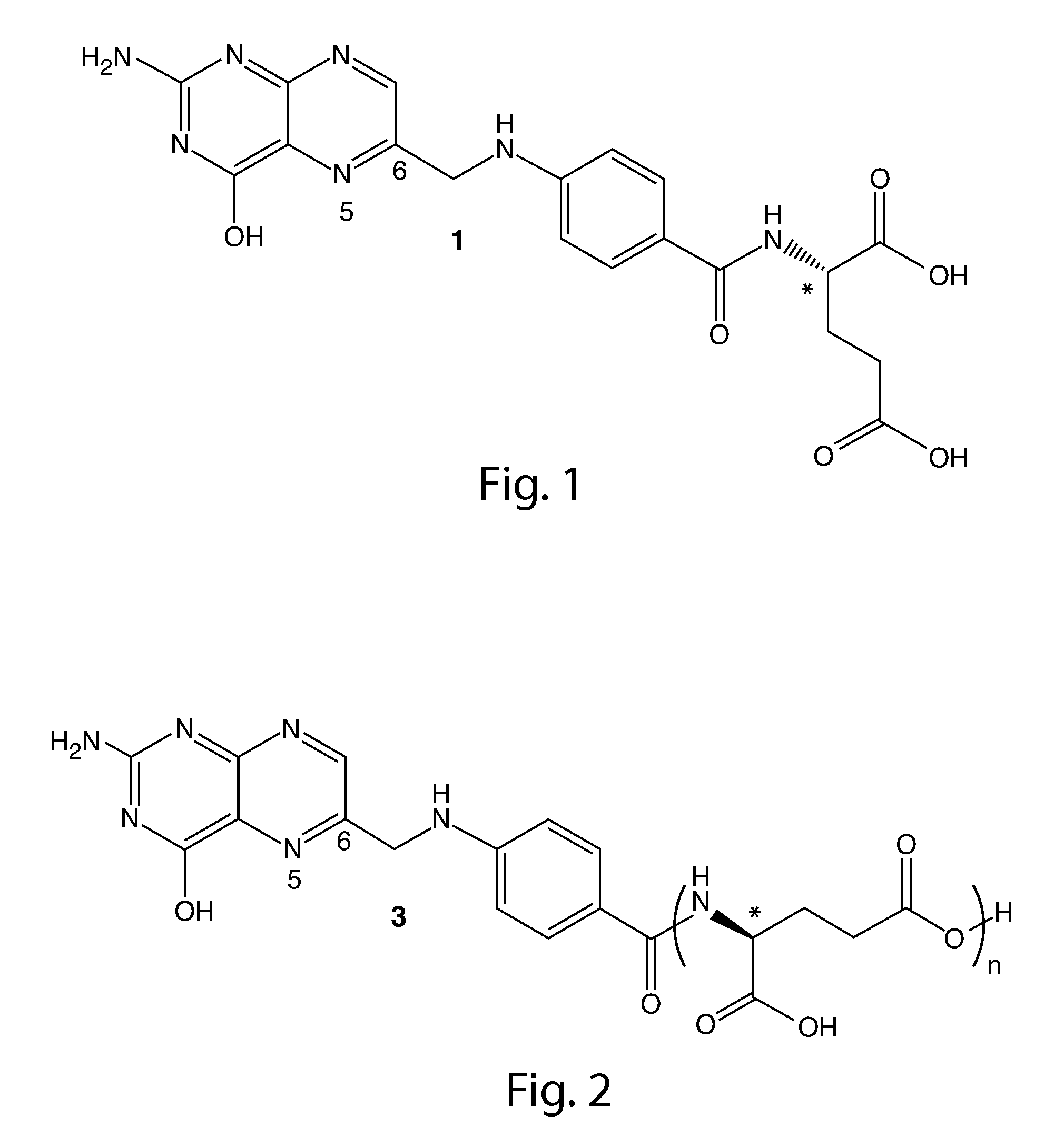
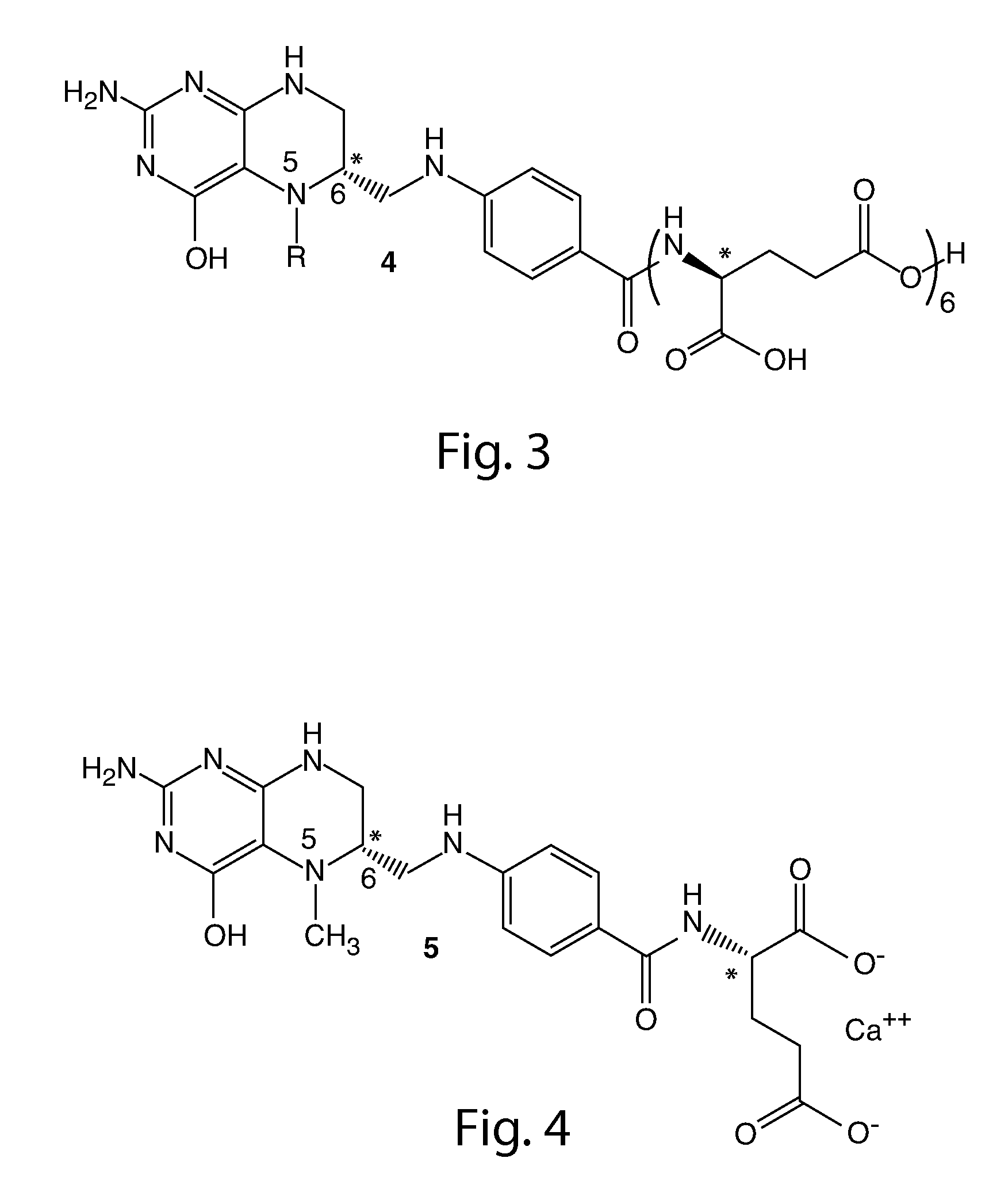
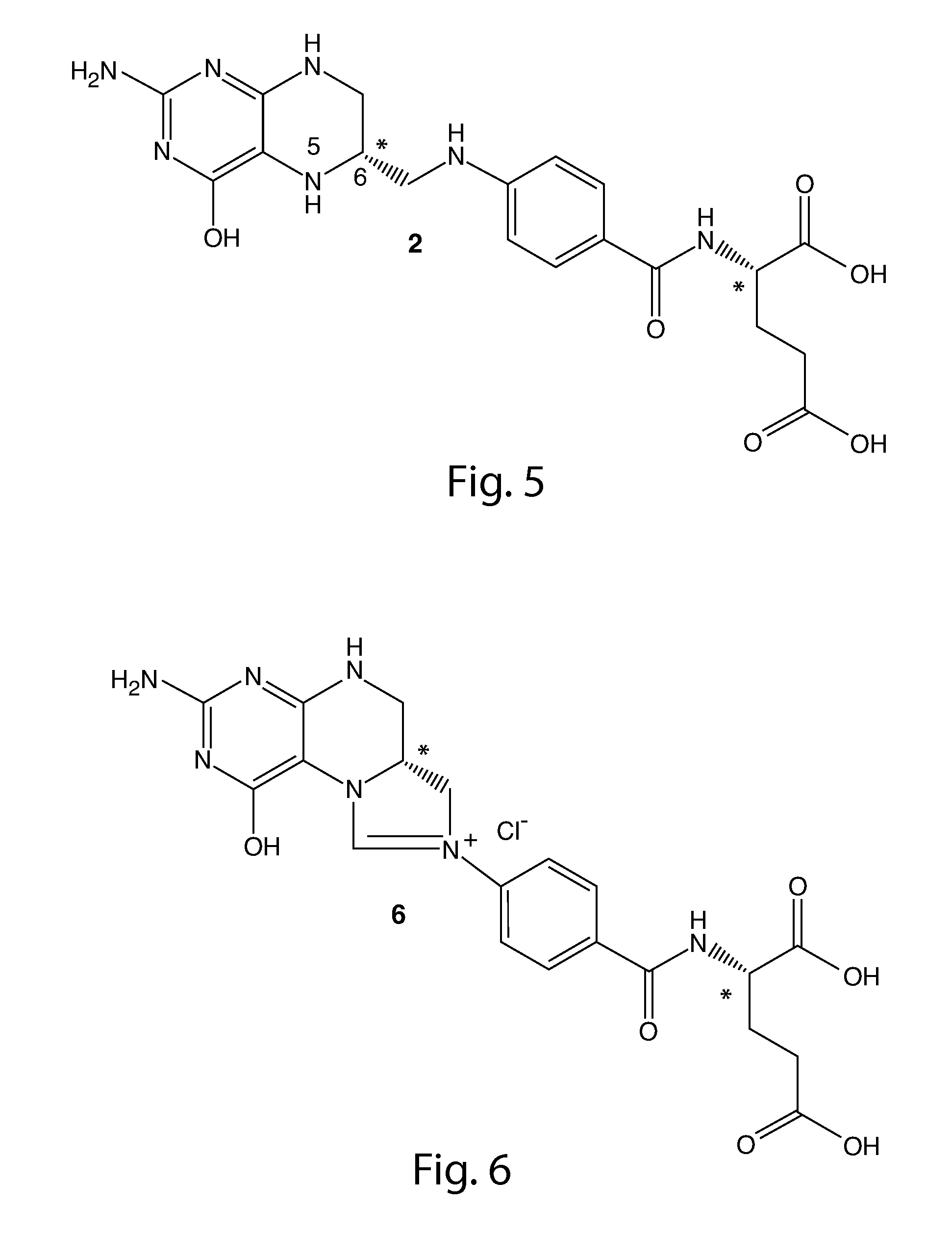
Synthesis of (6S)-5methyl-5,6,7,8-tetrahydrofolic acid
a technology of tetrahydrofolic acid and synthesis method, which is applied in the field of tetrahydrofolic acid synthesis and synthesis, can solve the problems of insufficient folate levels during pregnancy, human folate deficiency, and inability to synthesize folic acid, and achieve the effect of reducing dhfa and reducing folic acid (fa)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
[0038]In one preferred embodiment of the present invention, there is provided a one-pot process for the large-scale chemoenzymatic production of (6S)-5-methyl-5,6,7,8-tetrahydrofolic acid, also known as (6S)-5-methylTHFA. In this preferred form of the invention, the process comprises the steps of:
[0039](1) reduction of commercially available folic acid (FA) with zinc powder under basic conditions to give dihydrofolic acid (DHFA);
[0040](2) stereoselective reduction of DHFA with dihydrofolate reductase (DHFR) in the presence of NADP and an NADPH recycling system, i.e., glucose / GluDH, to give (6S)-THFA;
[0041](3) in situ conversion of (6S)-THFA to (6S)-5-methylTHFA by conventional methods; and
[0042](4) isolation of (6S)-5-methylTHFA as its calcium salt.
[0043]More particularly, in the first step, in an appropriately-sized reaction vessel, commercially-available FA is reduced to DHFA by stirring a basic solution (e.g., pH 13.5) of FA, sodium hydroxide (NaOH), and zinc powder for approxima...
second embodiment
[0050]The following process may also be used for the large-scale chemoenzymatic production of (6S)-5-methyl-5,6,7,8-tetrahydrofolic acid, also known as (6S)-5-methylTHFA. Accordingly, in another preferred embodiment, there is described a one-pot process for the large-scale chemoenzymatic production of (6S)-5-methyl-5,6,7,8-tetrahydrofolic acid (or 5-MeTHFA), which comprises the following four discrete steps:
[0051](1) reduction of commercially available folic acid (FA) with zinc powder under basic conditions to give dihydrofolic acid (DHFA);
[0052](2) stereoselective reduction of DHFA with dihydrofolate reductase (DHFR) in the presence of NADP and an NADPH recycling system, i.e., glucose / glucose dehydrogenase (GluDH), to yield (6S)-THFA;
[0053](3) in situ conversion of (6S)-THFA to (6S)-5-methylTHFA by conventional methods; and
[0054](4) isolation of (6S)-5-methylTHFA as its calcium salt.
[0055]The process of the second embodiment of the present invention is illustrated in FIG. 7.
[0056]M...
third embodiment
[0064]The following process may also be used for the large-scale chemoenzymatic production of (6S)-5-methyl-5,6,7,8-tetrahydrofolic acid, also known as (6S)-5-methylTHFA.
[0065]Accordingly, in the first step, folic acid (FA) (10 g) is suspended in water (50 mL) and concentrated ammonium hydroxide (3.2 mL) is added to dissolve the solid and attain a pH of 8.1. To this, a solution having a pH of 8 and containing ascorbic acid (10 g), concentrated ammonium hydroxide (4.9 mL) and water (50 mL) is then added. The pH of the resulting solution is approximately 8. This solution is then stirred under N2 and heated to a temperature of 60° C. To this mixture is added sodium dithionite (20 g). The pH of the resulting solution is 5.9. This solution is allowed to stir under N2, at 60° C. for 30 minutes. The pH is adjusted to 3 by the addition of concentrated hydrochloric acid (13.5 mL). To this mixture is added methanol (400 mL). This mixture is allowed to stir for 15 minutes, and the solid precip...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com