Method for treating a pulmonary hypertension condition
a pulmonary hypertension and pulmonary arterial pressure technology, applied in the direction of biocide, cardiovascular disorder, drug composition, etc., can solve the problems of difficult heart pumping blood through the lungs to be oxygenated, extreme shortness of breath of patients with pulmonary arterial hypertension, and high pulmonary arterial pressure, so as to prolong the life of the subject, improve the prognosis, and reduce the effect of baselin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
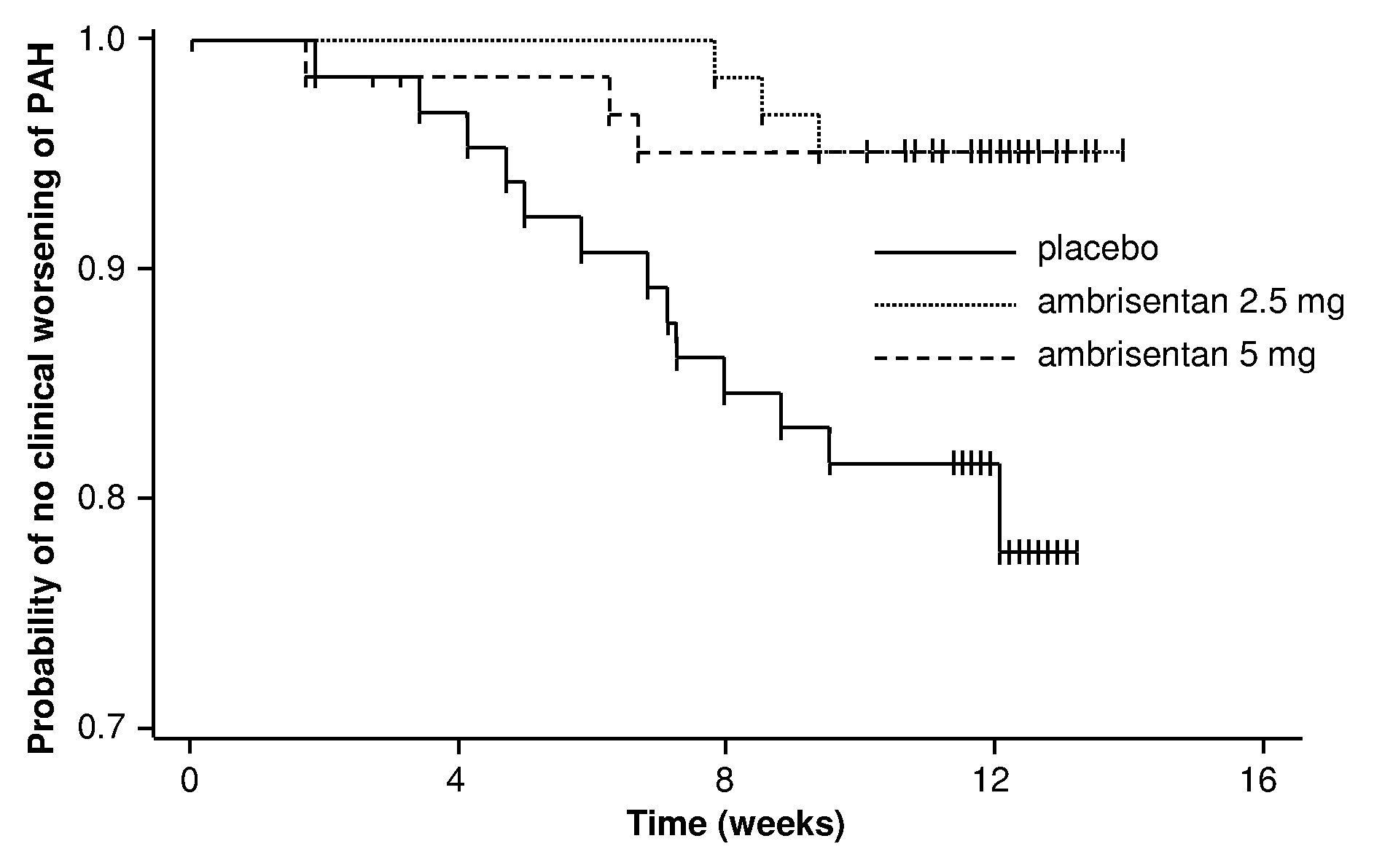
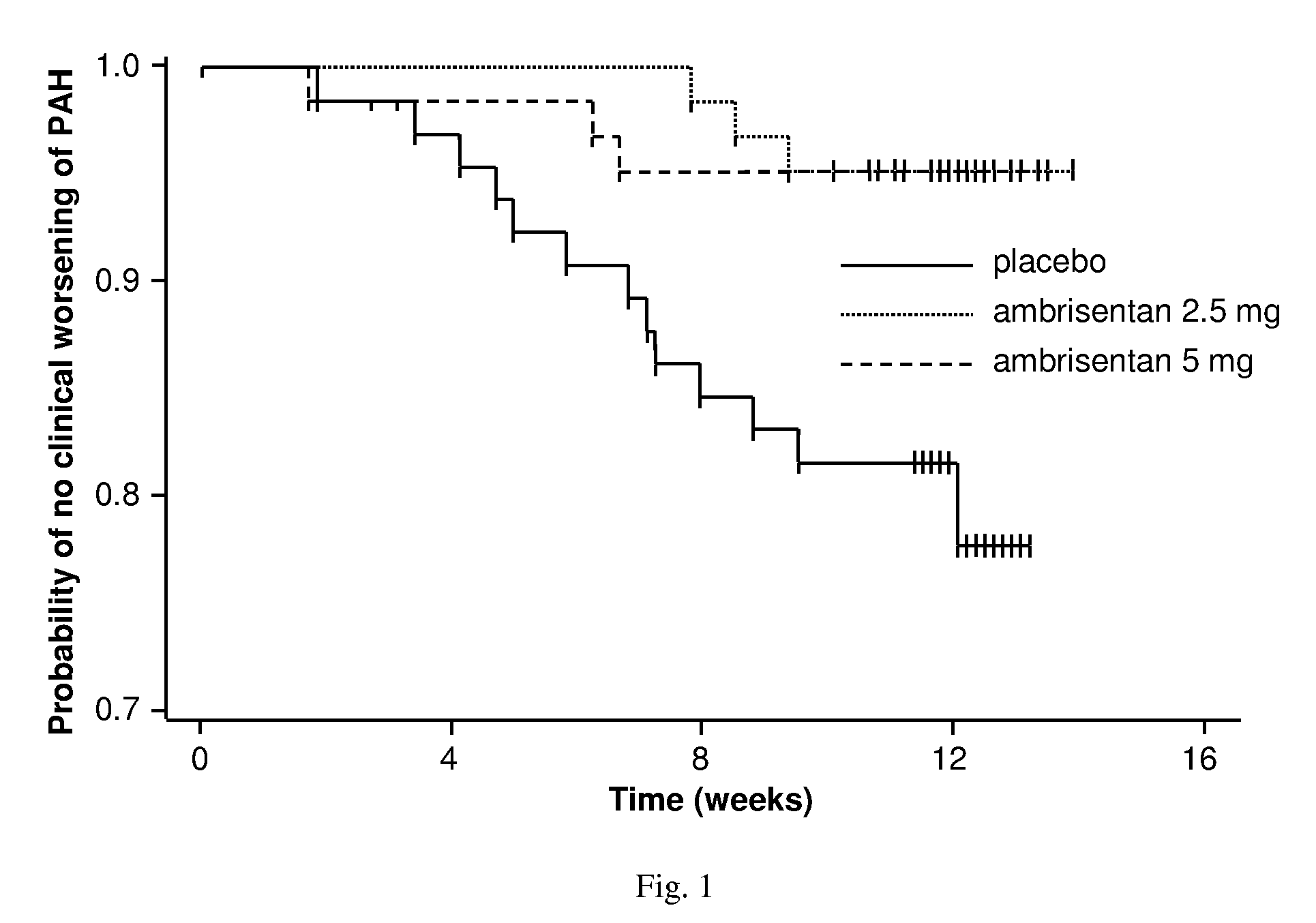
[0418]The ambrisentan treatment benefit observed by the primary and secondary endpoints of this study was robust, internally consistent, and clinically relevant.
[0419]Both doses demonstrated a statistically significant and clinically relevant improvement in 6MWD that was associated with a significant decrease in BDI. The improvement in 6MWD was nearly twice as large in the 5 mg dose group compared to the 2.5 mg dose group, and improvements in 6MWD were consistently dose-responsive for most subgroups evaluated. Furthermore, plasma BNP, a molecular marker that has been shown to decrease in patients with PAH who demonstrate improvements in 6MWD or hemodynamics, was substantially reduced with ambrisentan treatment. Ultimately, these symptomatic improvements resulted in a patient's self-assessment of an overall better quality of life, as measured by statistically significant improvements in several scales of the SF-36® health survey.
[0420]In addition to the symptomatic improvements obser...
example 2
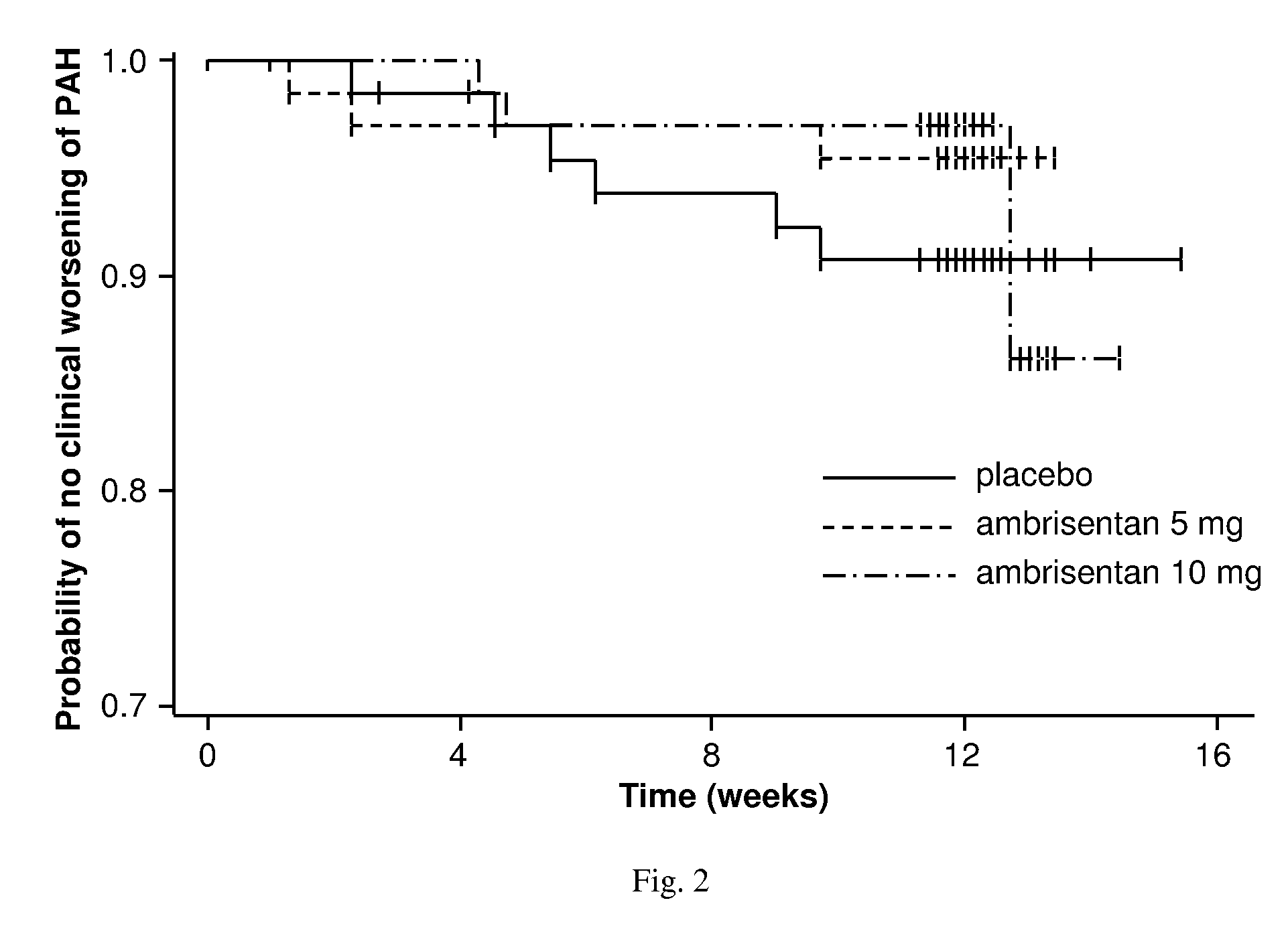
[0509]This study demonstrated that both the 5 mg and 10 mg dose of ambrisentan administered once daily provided statistically significant and clinically relevant improvements in exercise capacity and symptoms in subjects with PAH. The improvements in 6MWD were evident within 4 weeks and appeared dose-dependent by Week 8. At Week 12, the increase in 6MWD was nearly twice as large in the 10 mg dose group compared to the 5 mg dose group. Improvements in 6MWD were observed in most subgroups and, in general, appeared to be dose-dependent. Clinically relevant improvements in 6MWD were observed in subjects with WHO functional class I / II and class III / IV symptoms. Both doses also demonstrated clinically relevant treatment benefits for several secondary endpoints, including WHO functional class and BDI, as well as a notable reduction in plasma BNP.
[0510]Ambrisentan was well tolerated as indicated by the lack of dose reduction and AEs leading to premature discontinuation as well as more subje...
example 3
[0512]The trials described in Examples 1 and 2 enrolled subjects from a population having PAH including idiopathic PAH and PAH associated with CTD, anorexigen use or HIV infection. Patients with pulmonary hypertension due to other etiologies were generally excluded. However, the efficacy and safety of ambrisentan observed in this classic PAH population, and the need for effective therapy in pulmonary hypertension associated with other conditions, merits evaluation in these non-traditional groups. Therefore, a further study is conducted to evaluate safety and efficacy of ambrisentan in both classic PAH patients (WHO Group 1) and in an expanded pulmonary hypertension patient population (WHO Groups 3 and 4).
[0513]This expanded population includes subjects having idiopathic and familial PAH; PAH associated with collagen vascular disease, congenital systemic-to-pulmonary shunts, HIV infection, drugs and toxins, thyroid disorders, glycogen storage disease, Gaucher disease and splenectomy;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com