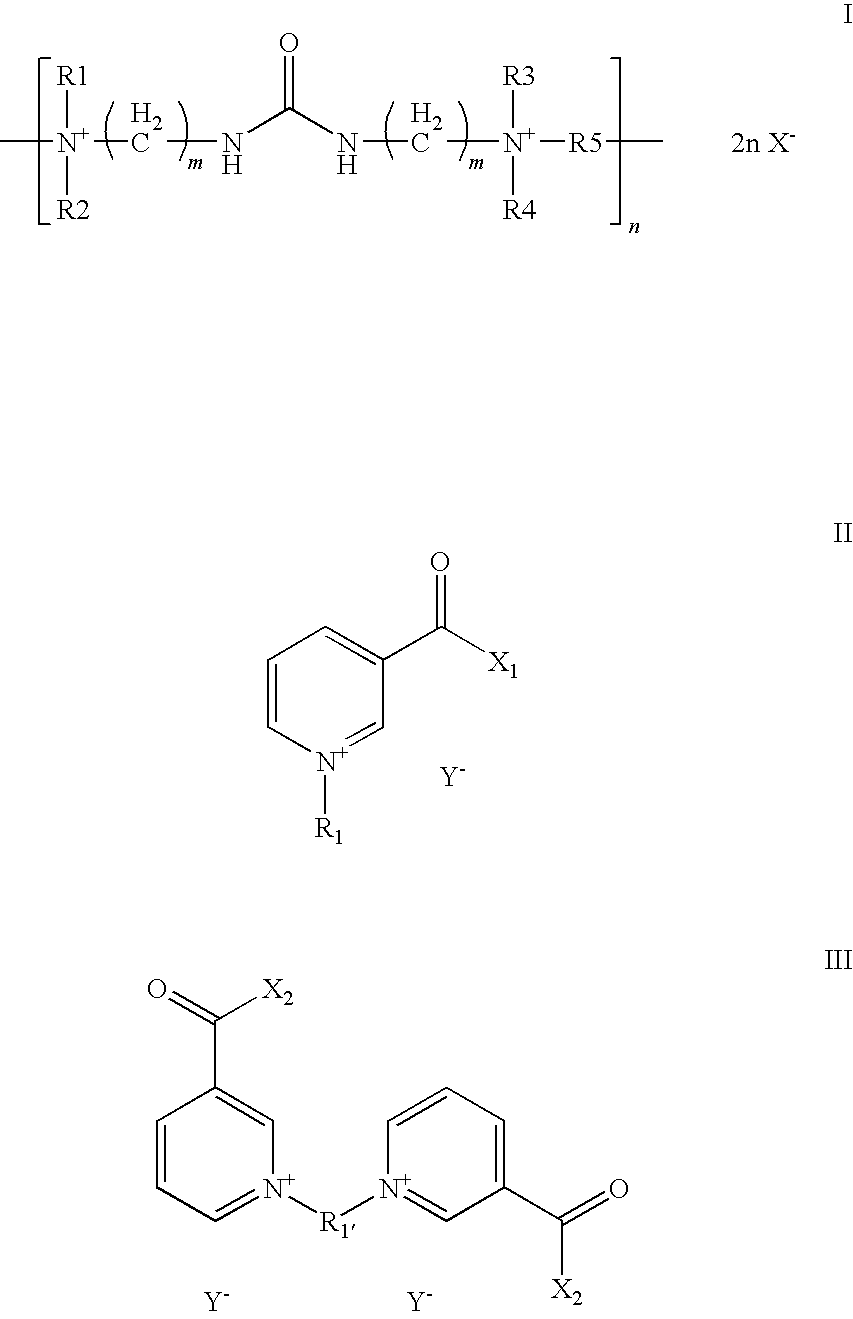
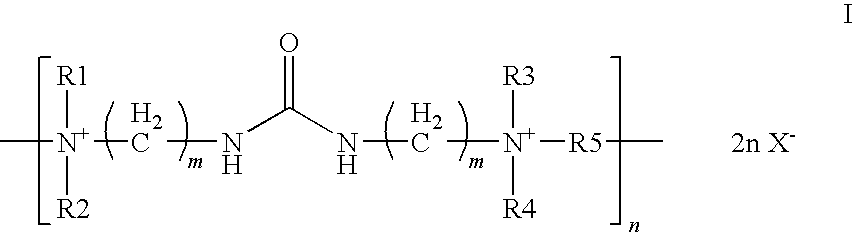
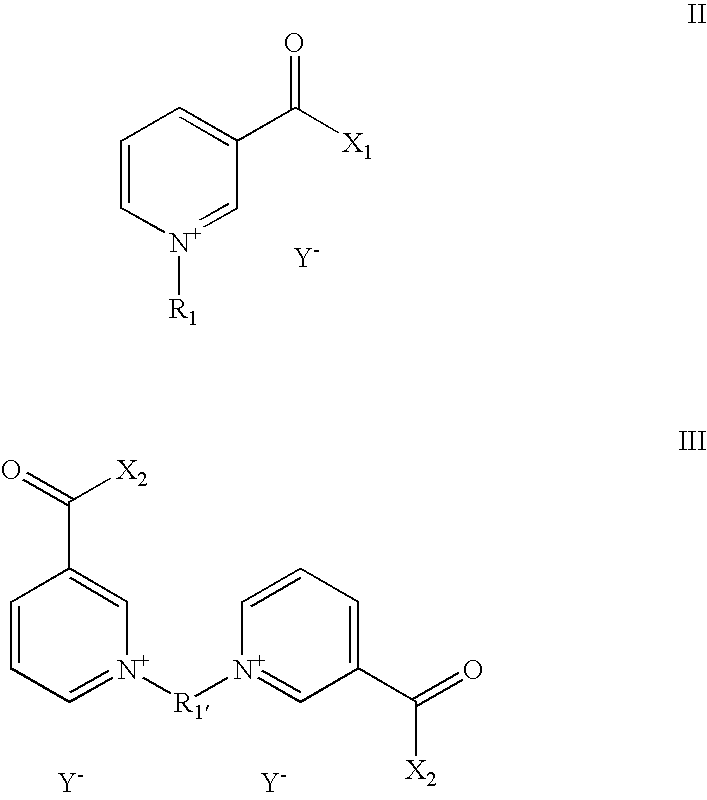
Aqueous,alkaline,cyanide-free bath for the galvanic deposition of zinc and zinc alloy coatings
a technology of zinc alloy coating and alkali bath, applied in the field of alkali galvanic bath, can solve the problems excessive thickness of zinc layers in areas of high current density, and uneven layer thickness distribution of zinc layers obtained according to these documents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
Synthesis of 1-(4′-methoxy-benzyl)-3-carbamoyl-pyridinium-chloride
[0066]In a 100 ml round-bottom flask with reflux condenser, 60 ml of water, 9.2 g of nicotinic acid amide (98%) (0.0738 mol), 11.68 g of 4-methoxybenzylchloride (99%) (0.07378 mol) are heated under reflux for 24 hours. After completion of the reaction, the water is removed in vacuo and the residue is taken up in 200 ml of ethanol and heated under reflux for another hour. The reaction mixture is then cooled to 4° C. and the white solid obtained is removed by filtration and dried in vacuo. This yielded 16.92 g of a white solid (82.26% of the theoretical yield).
preparation example 2
Synthesis of 1-(4′-chloro-benzyl)-3-carbamoyl-pyridinium-chloride
[0067]In a 100 ml round-bottom flask with reflux condenser, 60 ml of ethanol, 10 g of nicotinic acid amide (98%) (0.0802 mol), 13.05 g of 4-chloro-benzylchloride (99%) (0.0802 mol) are heated under reflux for 24 hours. After completion of the reaction, the solid residue is heated in an ethanol / methanol mixture for another 15 minutes and then cooled to 4° C. The solid obtained is removed by filtration and dried in vacuo. This yielded 18.82 g of a white solid (82.87% of the theoretical yield).
preparation example 3
Synthesis of 1,1′-(xylenyl)-3,3′-bis-carbamoyl-bis-pyridinium-dichloride
[0068]In a 100 ml round-bottom flask with reflux condenser, 60 ml of ethanol, 10 g of nicotinic acid amide (98%) (0.0802 mol), 7.16 g of α,α′-dichloro-p-xylene (98%) (0.0401 mol) are heated under reflux for 24 hours. After completion of the reaction, the solid residue is heated in 200 ml of an ethanol / methanol mixture for another 15 minutes and then cooled to 4° C. The resulting solid is removed by filtration and dried in vacuo. This yielded 12.29 g of a white solid (73.13% of the theoretical yield).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com