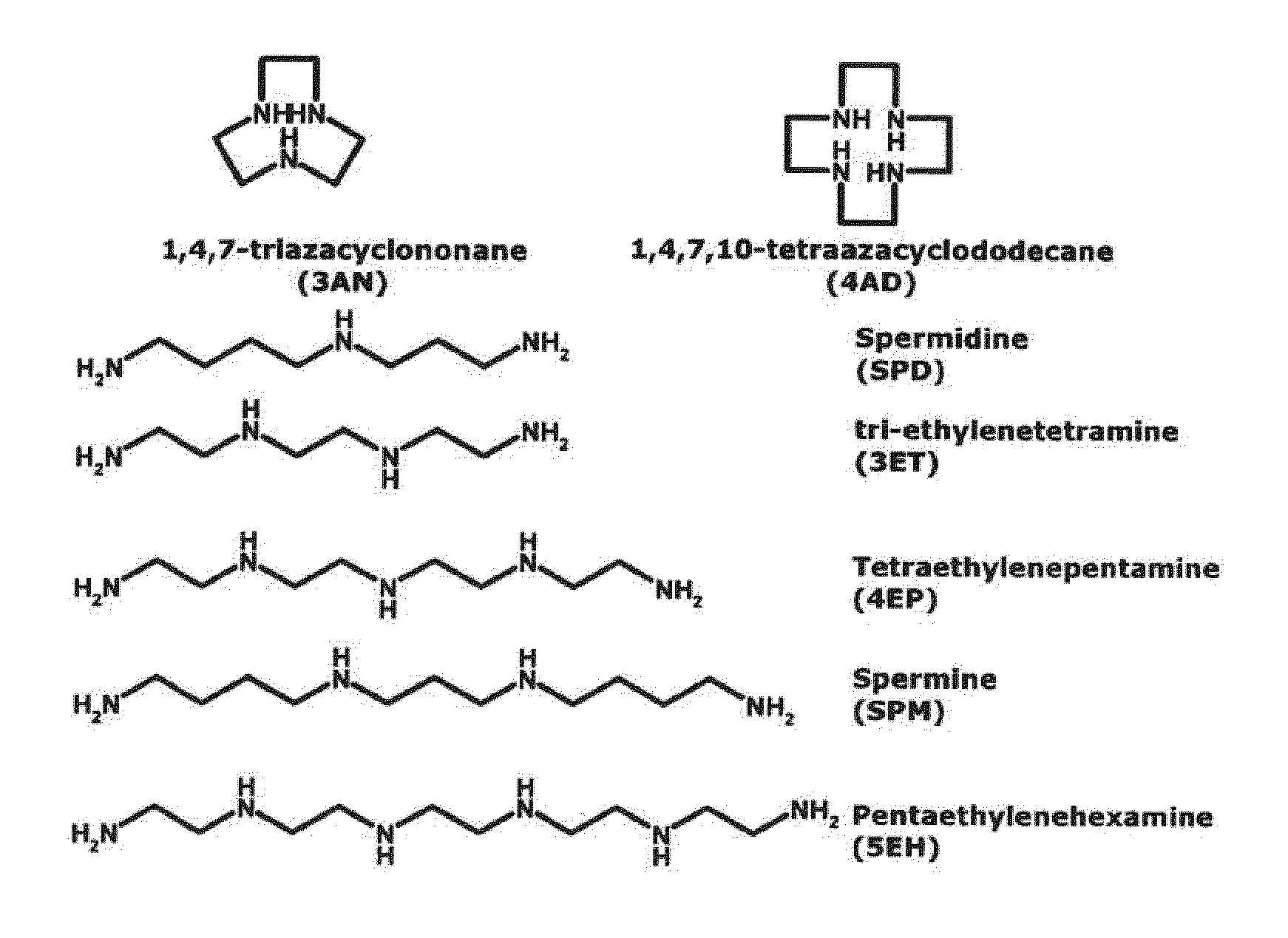
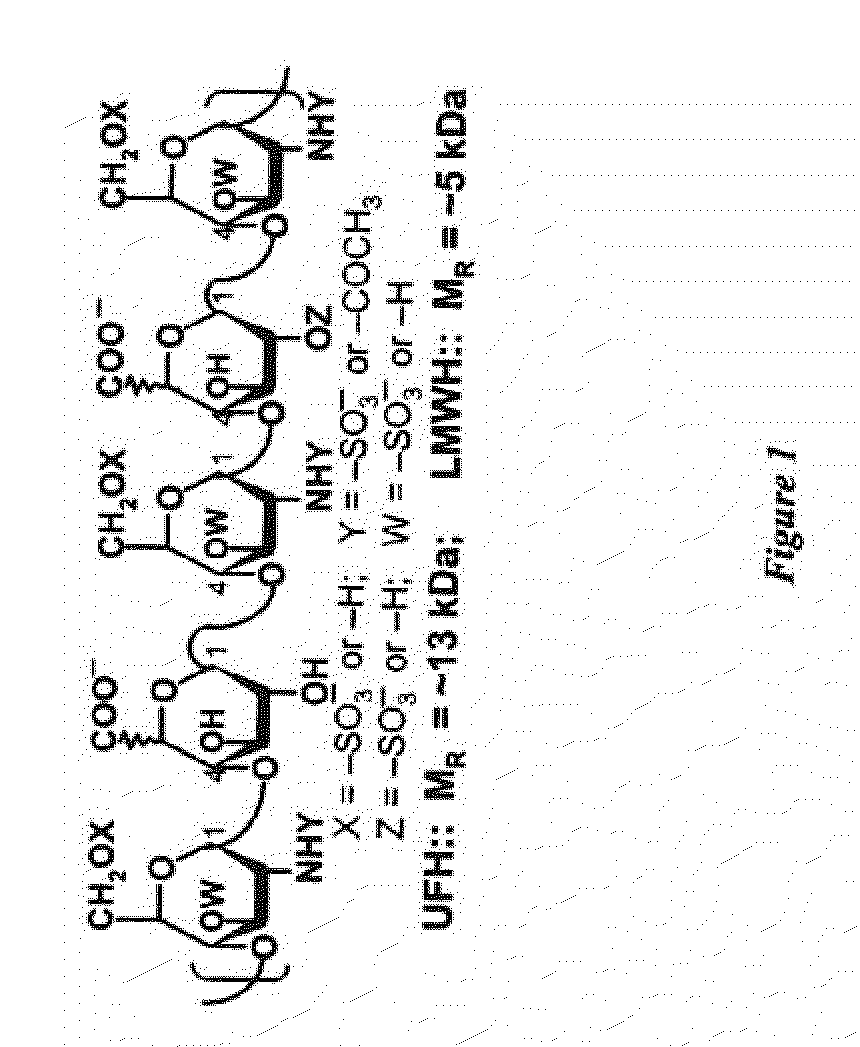
Method for finger-printing heparins
a fingerprinting and heparin technology, applied in the field of fingerprinting heparins, can solve the problems of increased bleeding risk, immunological reaction, patient-to-patient response variability, etc., and achieve the effect of accurate and consistent fingerprinting, high level of resolution, and polydisperse heparin mixtures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fingerprinting of Lovenox®
[0059]Three solutions were prepared. Solution A was 13.5 mg Lovenox® previously dialyzed to eliminate packaging excipients was dissolved in 260 μL of water; solution B was 25 mg of sodium cyanoborohydride in 300 μl of water; and solution C was 4 mg AMAC (FIG. 7) dissolved in 158 μL of 85% (v / v) acetic acid:DMSO. Solutions A, B and C were mixed and allowed to incubate at 37° C. for 16 hours. Following incubation, the mixture was dialyzed against high purity water to remove free, unreacted AMAC, followed by lyophilization. The solid powder so obtained was dissolved in high purity, deionized water containing 10% DMSO (v / v) at 10 mg / mL and stored at −78° C. until use.
[0060]CE was performed using a 75 μm fused silica capillary (40 cm effective length to the detector window) installed in a Beckman-Coulter P / ACE MDQ capillary electrophoresis system. A fresh capillary was activated using a 5 min flush of 1M NaOH, deionized water, 1M H3PO4, and deionized water each ...
example 2
Different LMWHs Display Different Fingerprint Patterns
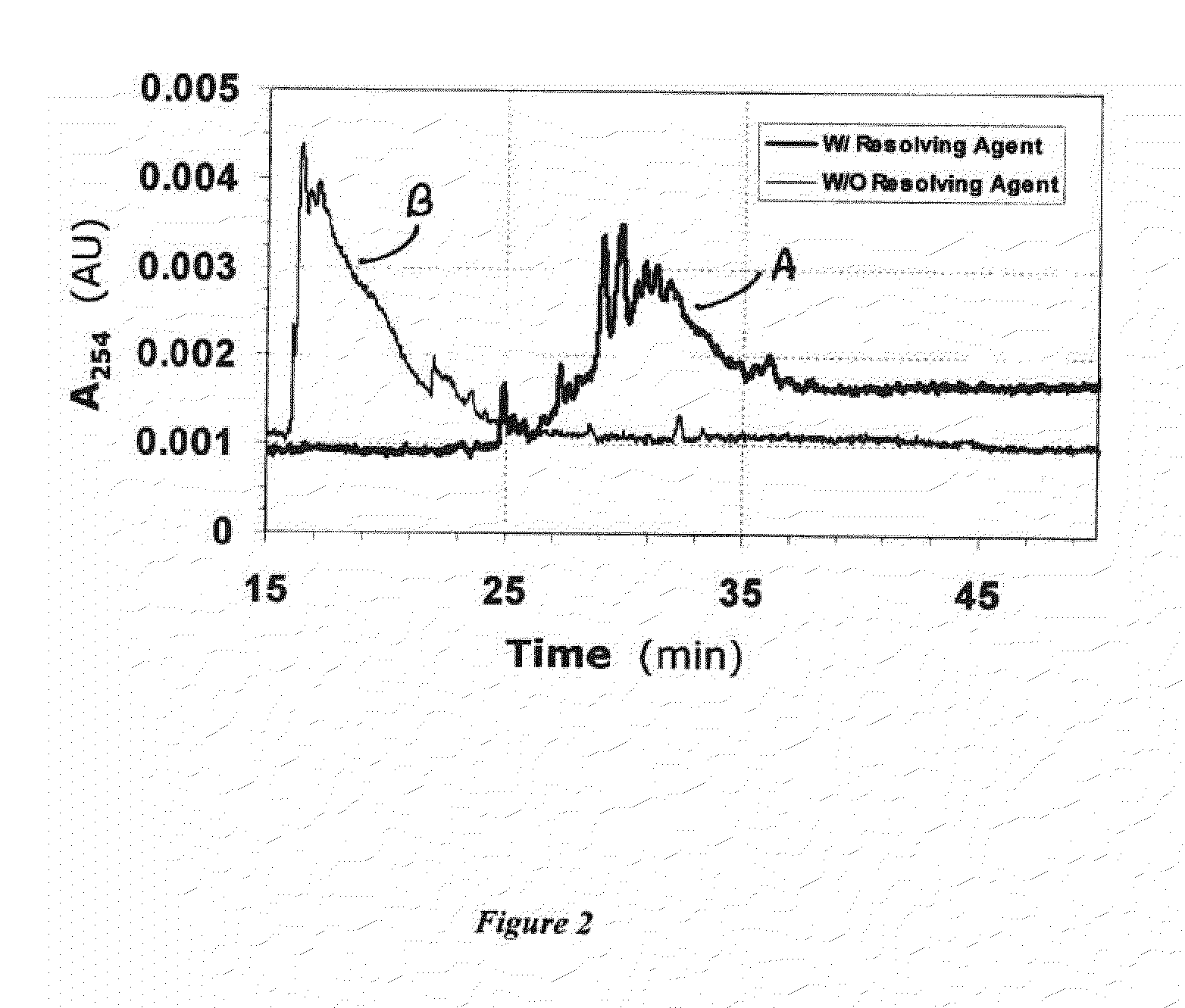
[0061]To assess whether fingerprint pattern is characteristic of individual LMWHs, we compared CE runs of enoxaparin, tinzaparin, and Sigma LMWH in the presence of 50 IM 4EP at pH 2.3 (FIG. 9). As can be seen, each LMWH shows a characteristic fingerprint pattern defined primarily by the extent of interaction with the resolving agent. Whereas enoxaparin displays prominent peaks at 25 and 30 min, Sigma LMWH is devoid of the pattern at 30 min. In contrast, both these patterns are absent in tinzaparin. Also, tinzaparin displays much lower resolution than enoxaparin and Sigma LMWH. Equivalent results were observed for other resolving agents including SPM and 5EH (not shown).
example 3
Consistency of Results Over Time
[0062]In this experiment, a generic form of LMWH from Sigma (labeled as Sigma LMWH) was used. As in Example 1, three solutions were prepared. Solution A was 35 mg Sigma LMWH (ID# H-3400), without pre-dialysis, dissolved in 500 μL of water; solution B was 63 mg of sodium cyanoborohydride in 750 μL of water; and solution C was 10 mg AMAC in 400 μL of 85% (v / v) acetic acid:DMSO. Solutions A, B, and C were mixed and allowed to incubate at 37° C. for 16 hours. Following incubation, the mixture was dialyzed and lyophilized in a manner identical to that described in Example 1. The solid powder so obtained was dissolved in high purity, deionized water to form a 10 mg / ml stock solution and stored −78° C. until use.
[0063]Three consecutive runs were performed to assess reproducibility of the electrophoretic profile. The fingerprints are shown in FIG. 10. As can be seen, the fingerprints were highly reproducible from run to run with an intraday variation of less ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric charge | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Mass ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com