Reparative cell delivery via hyaluronic acid vehicles
a technology of hyaluronic acid and reparative cells, applied in the direction of skeletal/connective tissue cells, prosthesis, drug compositions, etc., can solve the problems of the inability to apply the above mentioned advances in the field of reparative and regenerative cell populations, and the inability to achieve the functional restoration of compromised tissues and organs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
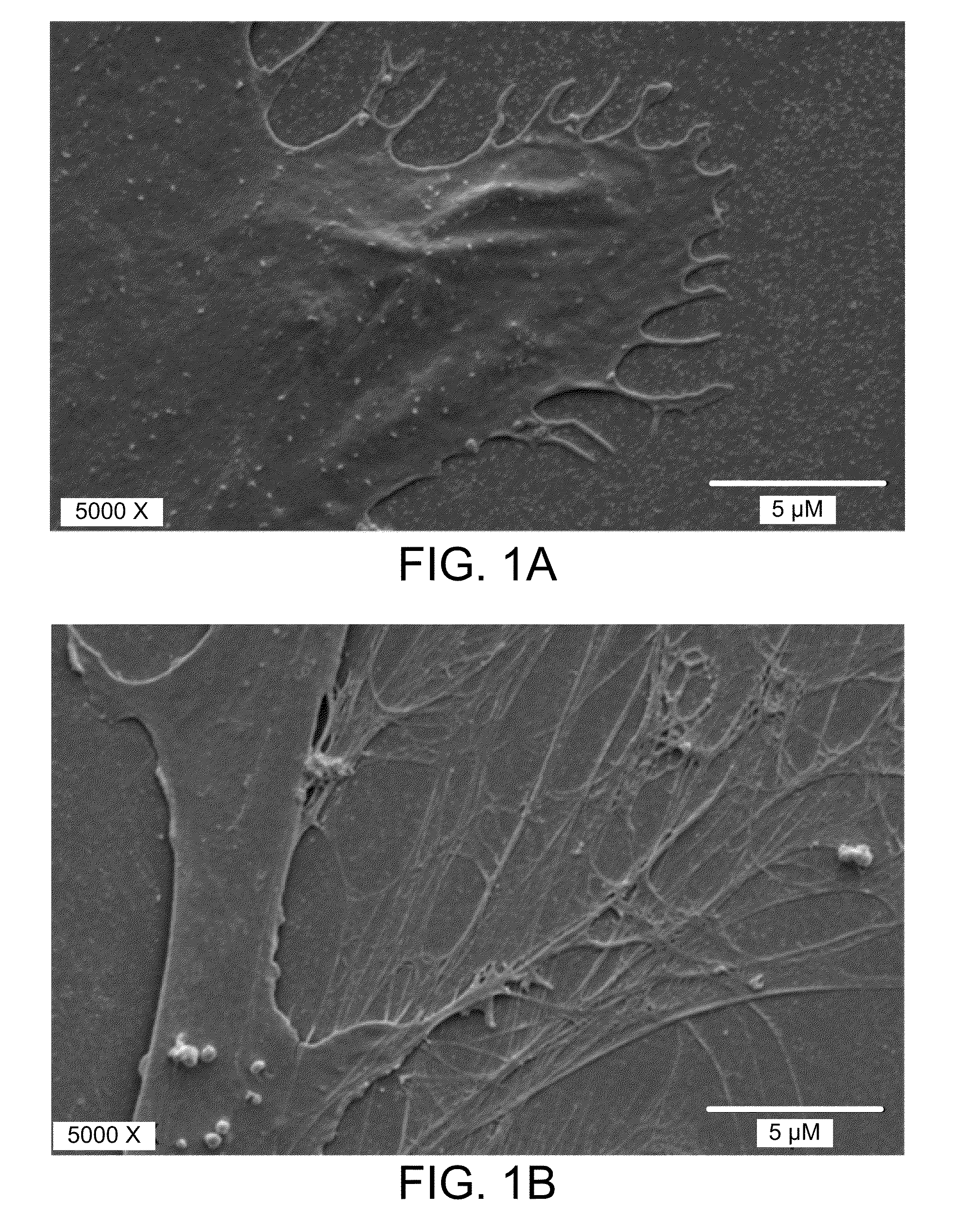
[0041]To explore the potential for a role for SVF cell in soft-tissue repair, the present inventors investigated the ability of the ADSC subset of SVF cells to proliferate and differentiate when exposed to hyaluronic acid compared with growth of ADSC under standard culture conditions. It was also investigated whether the mixture had a collagen-inducing effect in vitro.
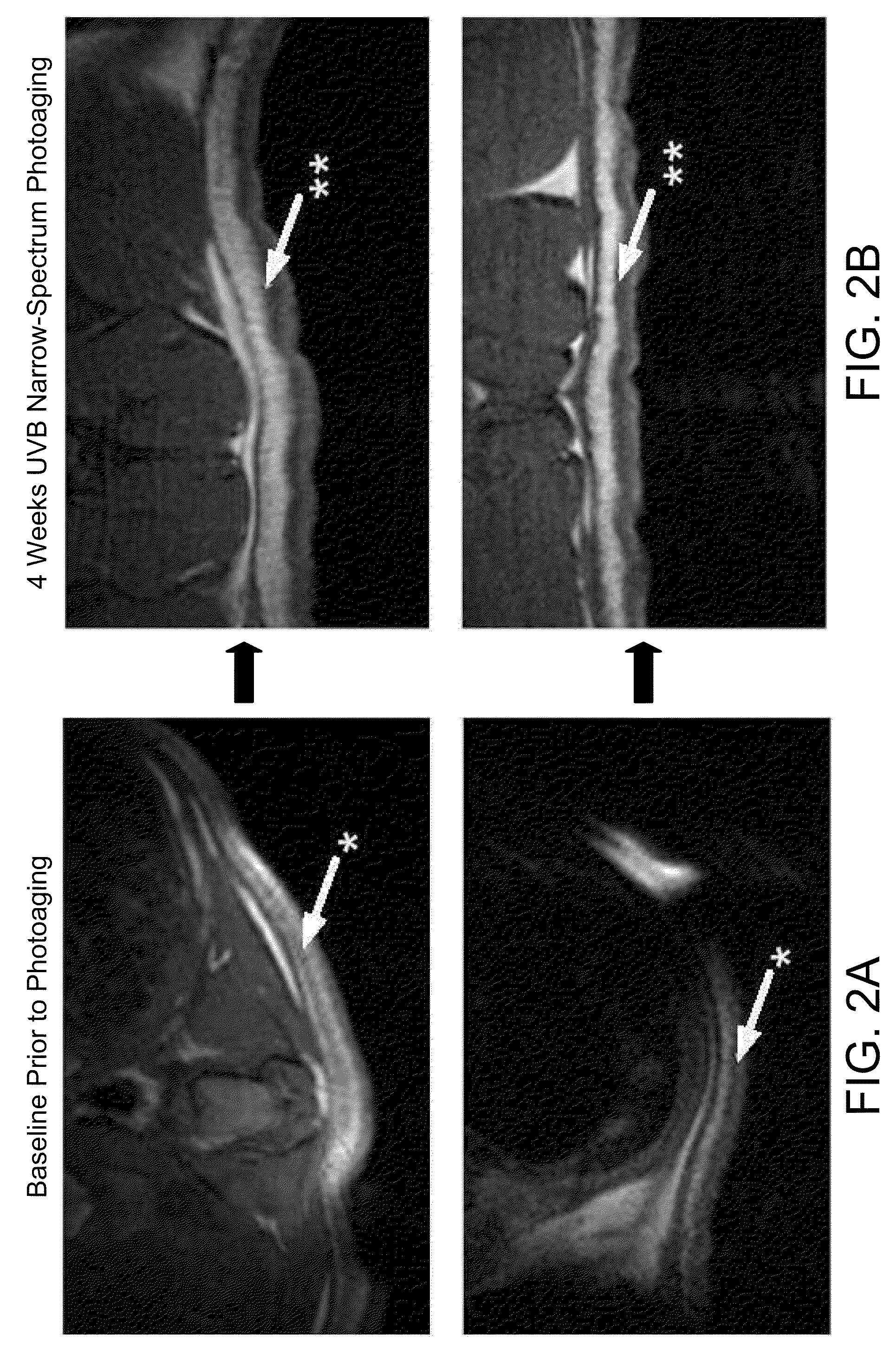
[0042]As disclosed herein, the present inventors have demonstrated that a commonly used injectable soft-tissue NASHA filler is compatible with tissue-resident MSCs derived from adipose tissue. The ADSCs were shown to proliferate equally when grown on HA or NASHA substrates compared to standard tissue culture-conditions on a plastic substrate. Real-time PCR showed that procollagen gene expression is increased in ADSC cultures in conjunction with HA. Importantly, in vivo studies showed a significant increase in vascular density at the site of ASC-NASHA grafts was shown as compared with NASHA alone. A robust vascular supp...
example 2
[0065]It has been shown that the phenotype of plastic adherent adipose derived cells changes with cell culture and is influenced by culture conditions. (Gimble J and Guilak F. “Adipose-derived adult stem cells: isolation, characterization, and differentiation potential”Cytotherapy 5(5) (2003) 362-369; Boquest A C, et al “Isolation and transcription profiling of purified uncultured human stromal stem cells: Alteration of gene expression after in vitro cell culture”Mol. Biol. Cell 16(3) (2005) 1131-1141). Use of reparative cell preparations such as freshly derived SVF populations for incorporation into HA fillers would avoid the need for culture with its attendant disadvantages and would permit utilization of larger cell numbers including cells that would ultimately exhibit the properties of plastic adherent stem cells if given the chance but are discarded using standard stem cell isolation procedures that call for the discard of all cells that are not adherent at 24 hours of culture....
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| molecular-weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com