Methods of depositing discrete hydroxyapatite regions on medical implants
a technology of hydroxyapatite and medical implants, applied in the field of medical implants, can solve problems such as neogagging its
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0035]A disc of Ti6Al4V having a radius of about 0.5 inches and a thickness of 0.25 inches is prepared with a smooth machine finish, cleaned in an ultrasonic bath, and rinsed with distilled water. The thickness portion is masked and the disc sample is placed into an electrolyte solution at ambient temperature including 150 ml each of a stock solution of CaCl2 and NH4H2PO4 in concentrations of 33 mM and 20 mM, respectively. Deionized water is added providing a 3 L total volume solution having a final concentration of 1.67 mM calcium ions and 1.0 mM phosphate ions. The pH is adjusted to 5.1 using hydrochloric acid.
[0036]After connection to a potentiostat, electrochemical deposition is carried out by means of galvanostatic polarization under cathodic current flow at a current density of about 40 mA / cm2 in order to provide a high flux of ions toward the cathode. After a deposition time of about 2.5 minutes, the cathodic polarization is complete and the sample is removed and rinsed with ...
example 2
[0037]A disc of Ti6Al4V is prepared as in Example 1. The disc sample is placed into an electrolyte solution at ambient temperature including 15 ml each of a stock solution of CaCl2 and NH4H2PO4 in concentrations of 33 mM and 20 mM, respectively. Deionized water is added providing a 3 L total volume solution having a final concentration of 0.167 mM calcium ions and 0.1 mM phosphate ions. The pH is adjusted to 6.4 using ammonium hydroxide.
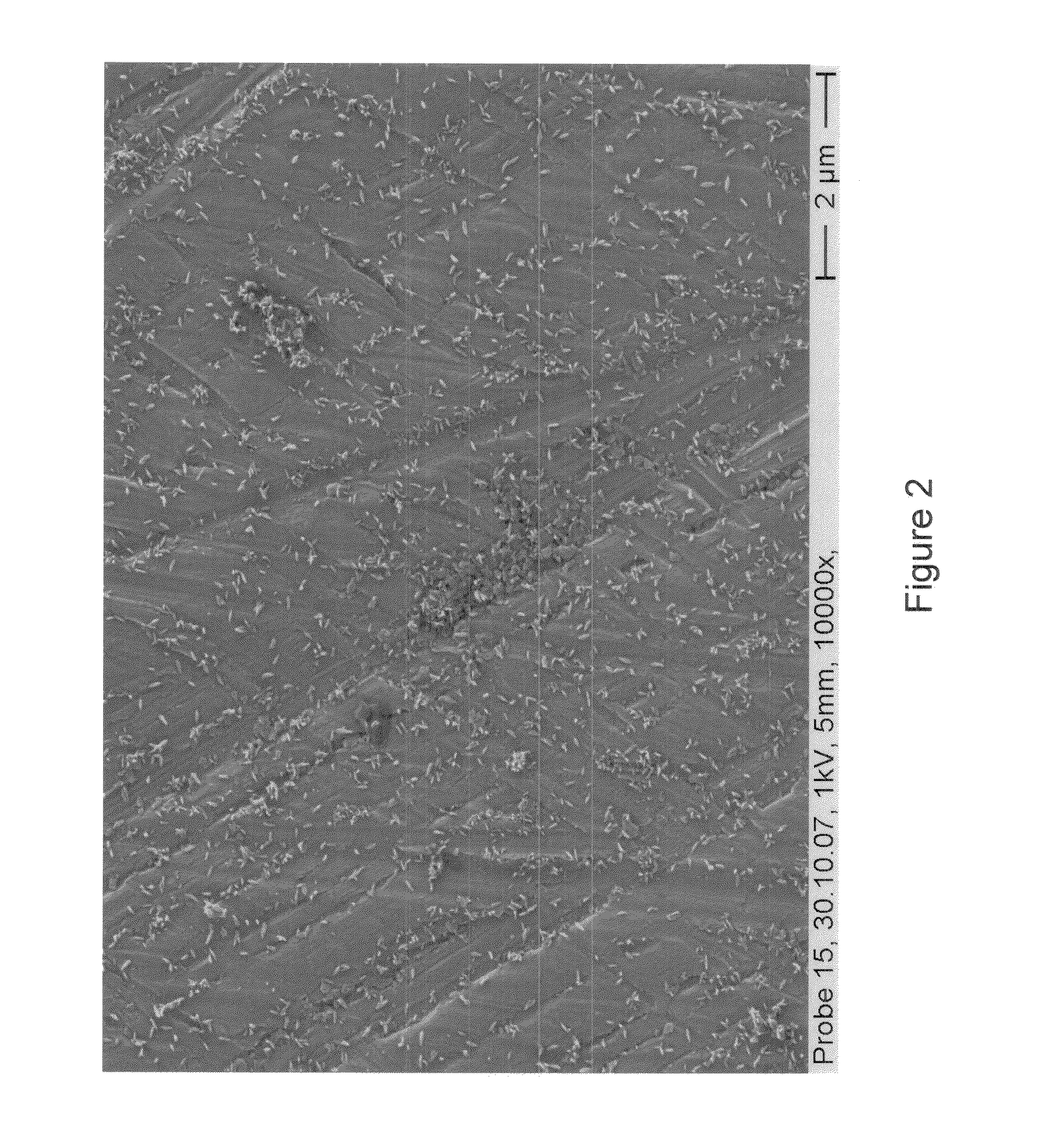
[0038]After connection to a potentiostat, electrochemical deposition is carried out by means of galvanostatic polarization under cathodic current flow at a current density of about 20 mA / cm2 in order to provide a high flux of ions toward the cathode. After a deposition time of about 10 minutes, the cathodic polarization is complete and the sample is removed and rinsed with deionized water. Electron microscopic examination reveals a plurality of discrete but homogenous regions of CPP having needle like morphology as shown in FIG. 2. Further IR-spectro...
example 3
[0039]A disc of Ti6Al4V is prepared as in Example 1. The disc sample is placed into an electrolyte solution at ambient temperature including 15 ml each of a stock solution of CaCl2 and NH4H2PO4 in concentrations of 33 mM and 20 mM, respectively. Deionized water is added providing a 3 L total volume solution having a final concentration of 0.167 mM calcium ions and 0.1 mM phosphate ions. The pH is adjusted to 6.4 using ammonium hydroxide.
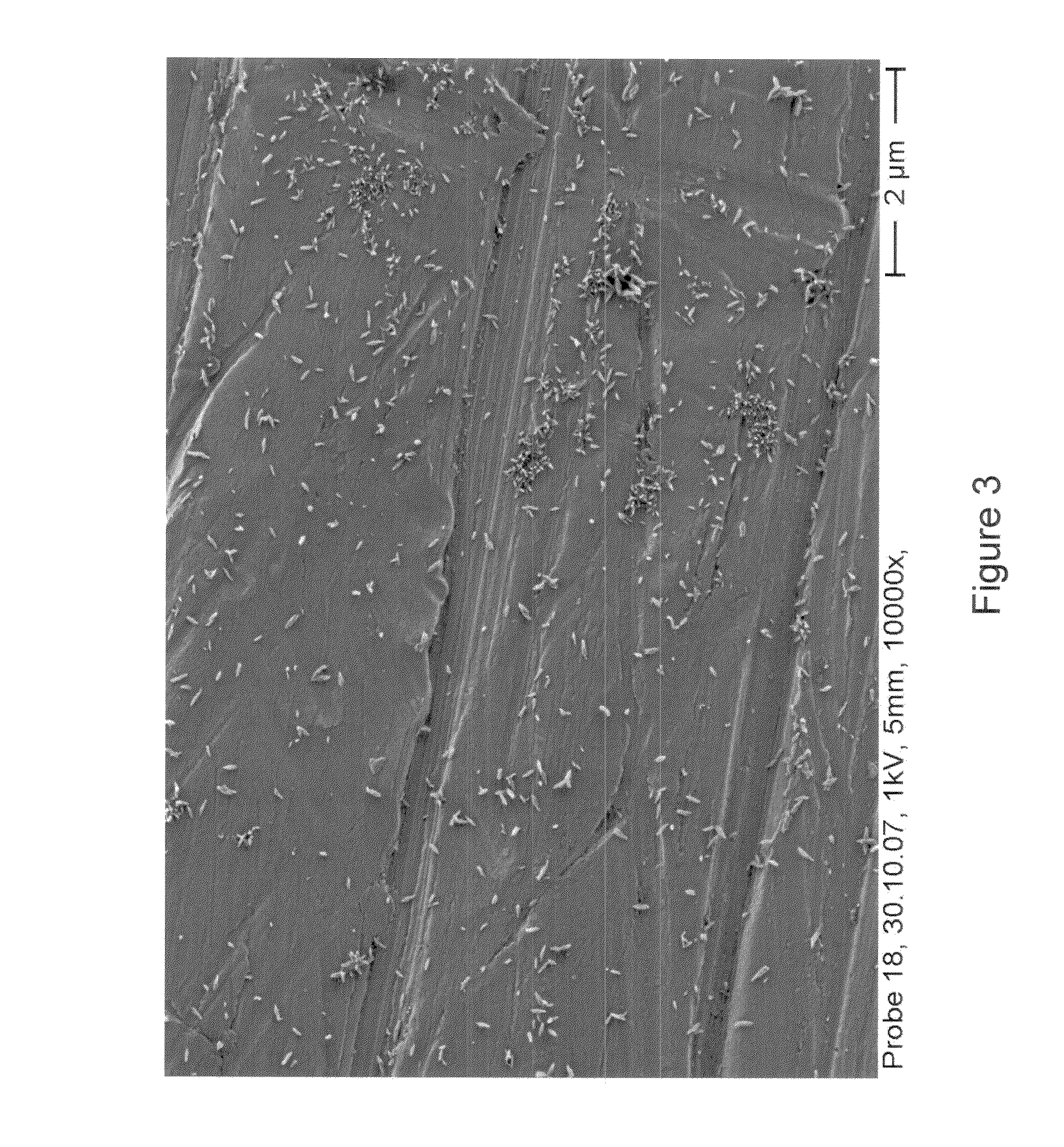
[0040]After connection to a potentiostat, electrochemical deposition is carried out by means of galvanostatic polarization under cathodic current flow at a current density of about 30 mA / cm2 in order to provide a high flux of ions toward the cathode. After a deposition time of about 10 minutes, the cathodic polarization is complete and the sample is removed and rinsed with deionized water. Electron microscopic examination reveals a plurality of discrete but homogenous regions of CPP having needle like morphology as shown in FIG. 3. Further IR-spectro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| constant current density | aaaaa | aaaaa |
| constant current density | aaaaa | aaaaa |
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com