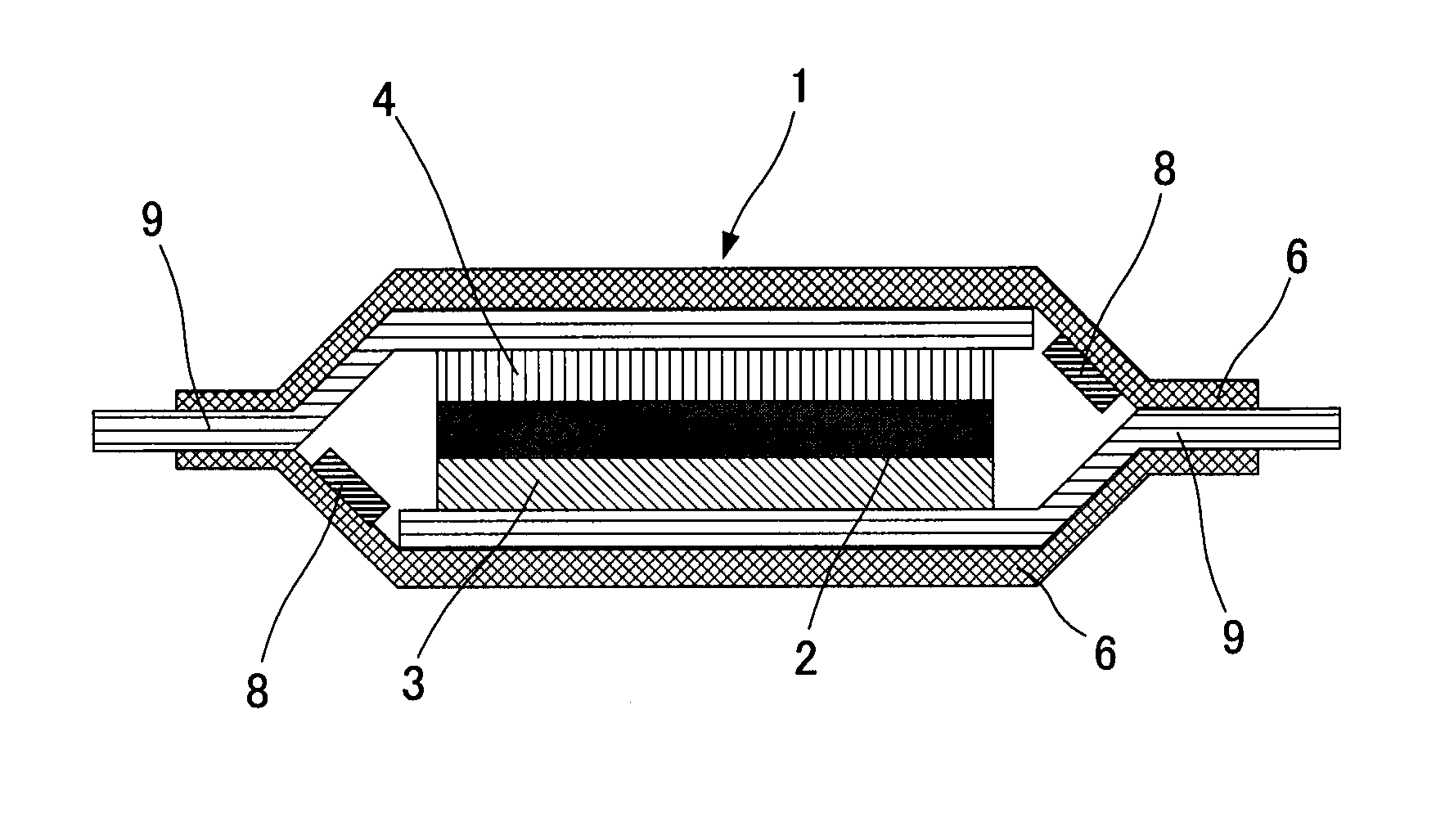
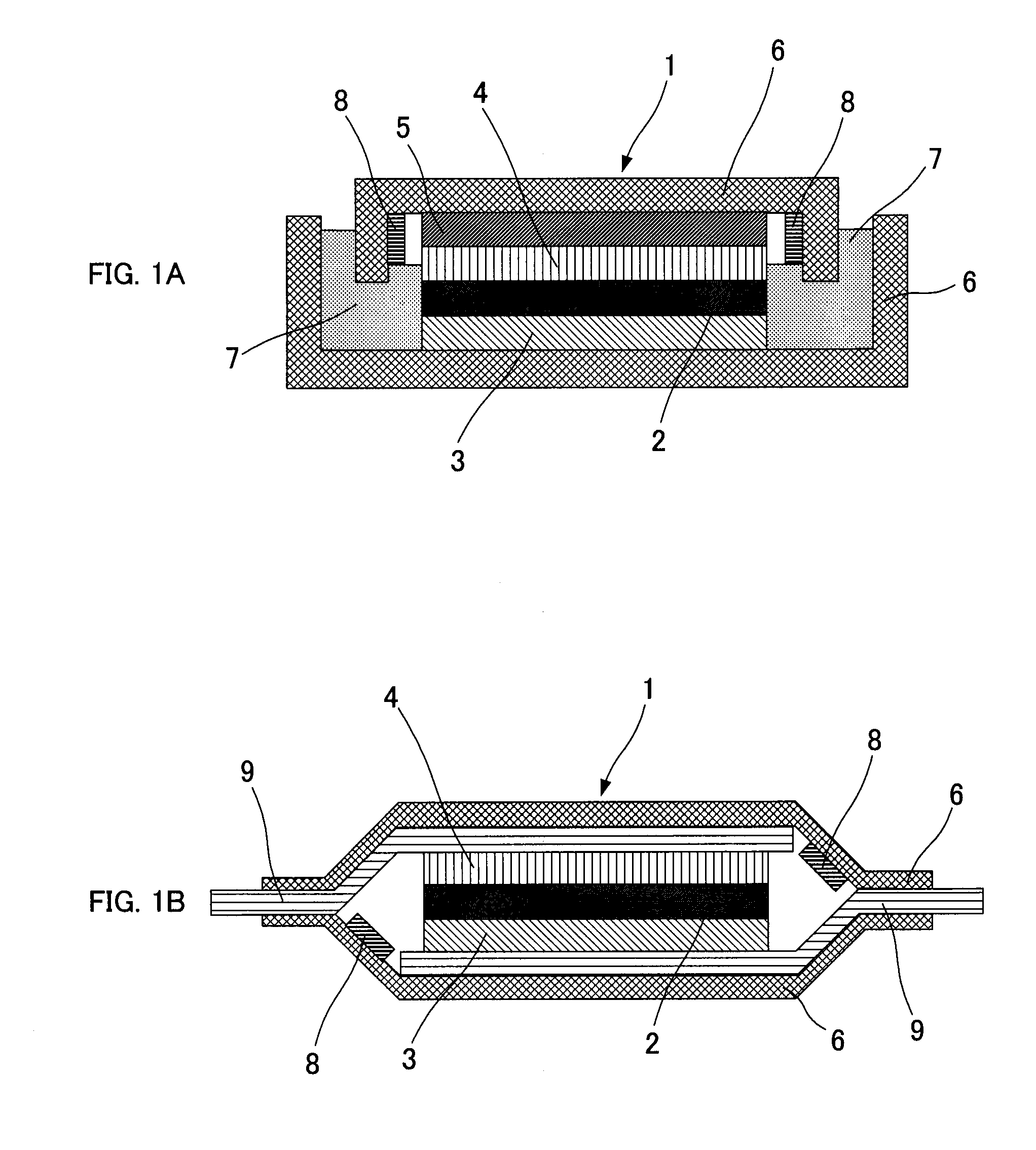
All-solid-state lithium secondary battery
a lithium secondary battery, all-solid-state technology, applied in the direction of electrochemical generators, cell components, cell component details, etc., can solve the problems of leaking hydrogen sulfide gas to the outside of the battery case, loss of adsorption capacity, and inability to prevent the leakage of hydrogen sulfide gas generated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of an All-Solid-State Lithium Secondary Battery
[0063]A cathode active material (LiCoO2) and a solid electrolyte material (LiGe0.25P0.75S4) were mixed by a mass ratio of 7:3 and a cathode mix was prepared. This cathode mix of 15 mg and the solid electrolyte material of 200 mg, and an indium foil of 60 mg (thickness 0.2 mm) as an anode were placed in a molding holder and pressed by 5 t / cm2 to produce an electrode pellet having a diameter of about 10 mm and a thickness of about 1.5 mm.
[0064]Next, an aqueous solution of cupric nitrate was dropped onto the end part of the inner side of an upper cover for a battery case of coin case type (made of SUS) and dried to precipitate cupric nitrate (metal salt) of about 0.5 g. Further, as the Example was supposed to create a submersion of the battery case at the time of case breakage, a hole of φ 1 mm was made to the upper cover of the coin case.
[0065]The above-mentioned electrode pellet was placed inside of the coin case and the coin ...
example 2
Production of an All-Solid-State Lithium Secondary Battery
[0066]A cathode active material (LiCoO2) and a solid electrolyte material (LiGe0.25P0.75S4) were mixed by a mass ratio of 7:3 and a cathode mix was prepared. This cathode mix of 15 mg and the solid electrolyte material of 200 mg, and an indium foil of 60 mg (thickness 0.2 mm) as an anode were placed in a molding holder and pressed by 5 t / cm2 to produce an electrode pellet having a diameter of about 10 mm and a thickness of about 1.5 mm.
[0067]Next, an aqueous solution of cupric nitrate was dropped onto an upper cover for a battery case of laminate case type (made of aluminum) provided with a current collector made of SUS and a part of the inside of a lower cover thereof where no current collector is provided, that is the part where no potential is applied, and dried to precipitate cupric nitrate (metal salt) of about 0.5 g. Further, as the Example was supposed to create a submersion of the battery case at the time of case brea...
example 3
[0069]A laminate cell was produced in the same manner as in the Example 2 except that a solid electrolyte material was changed to 70Li2S-30P2S5 (obtained by following to the method disclosed in JP-A No. 2005-228570, wherein Li2 and P2S5 were vitrified by a planetary ball mill with a mole ratio of Li2S:P2S5=70:30 and then by heat treated) and an amount of cupric nitrate precipitated was made to 1.0 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com