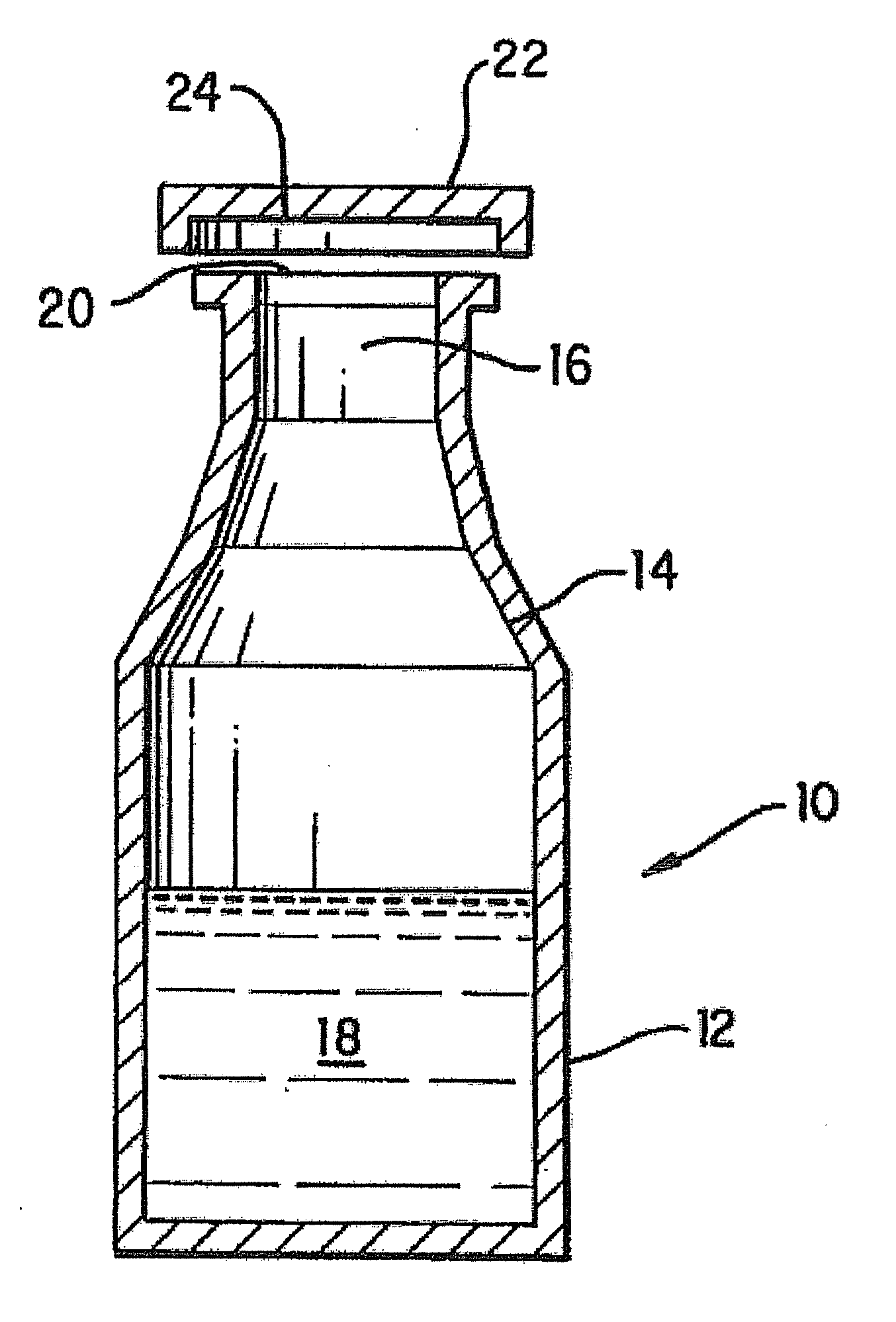
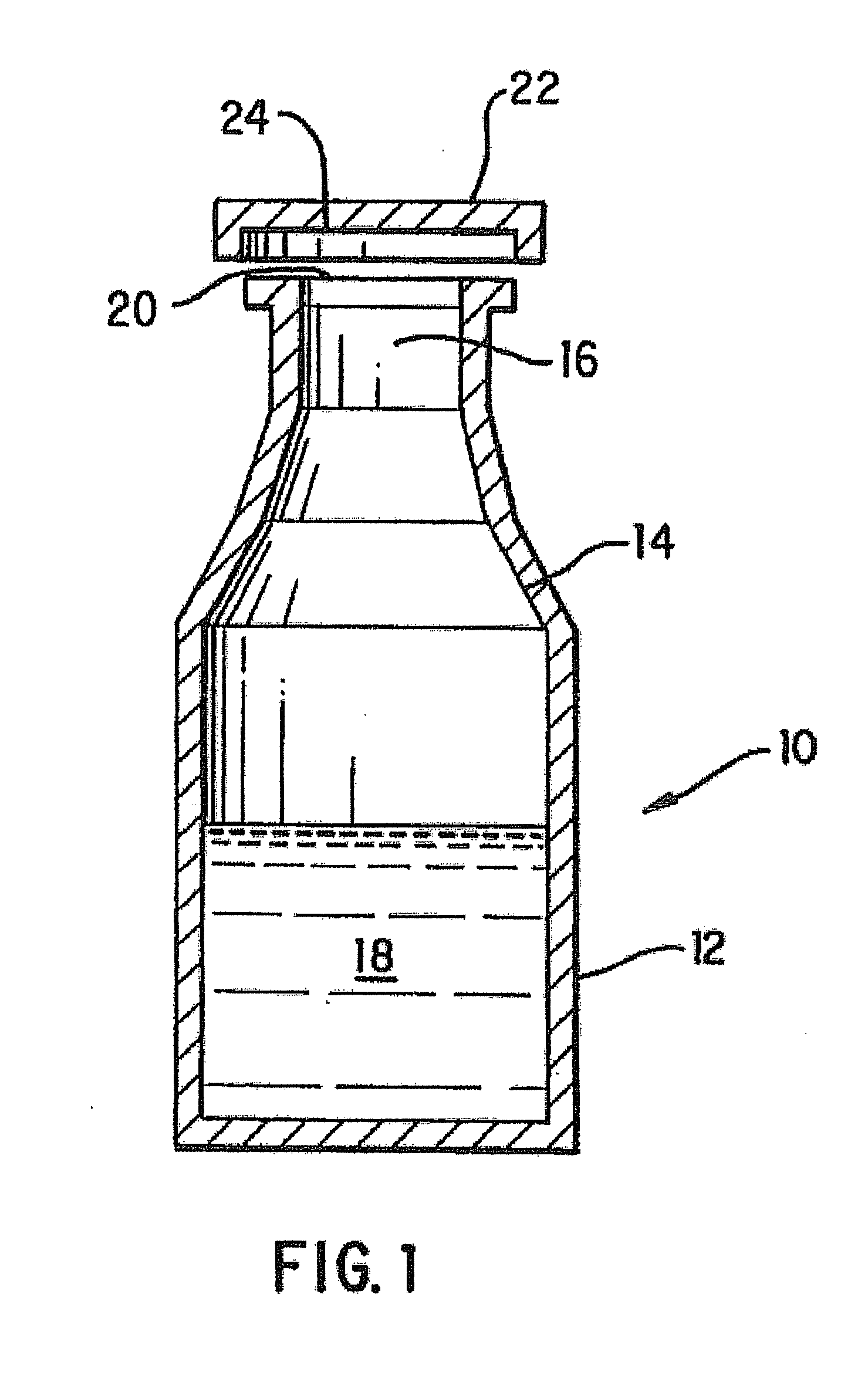
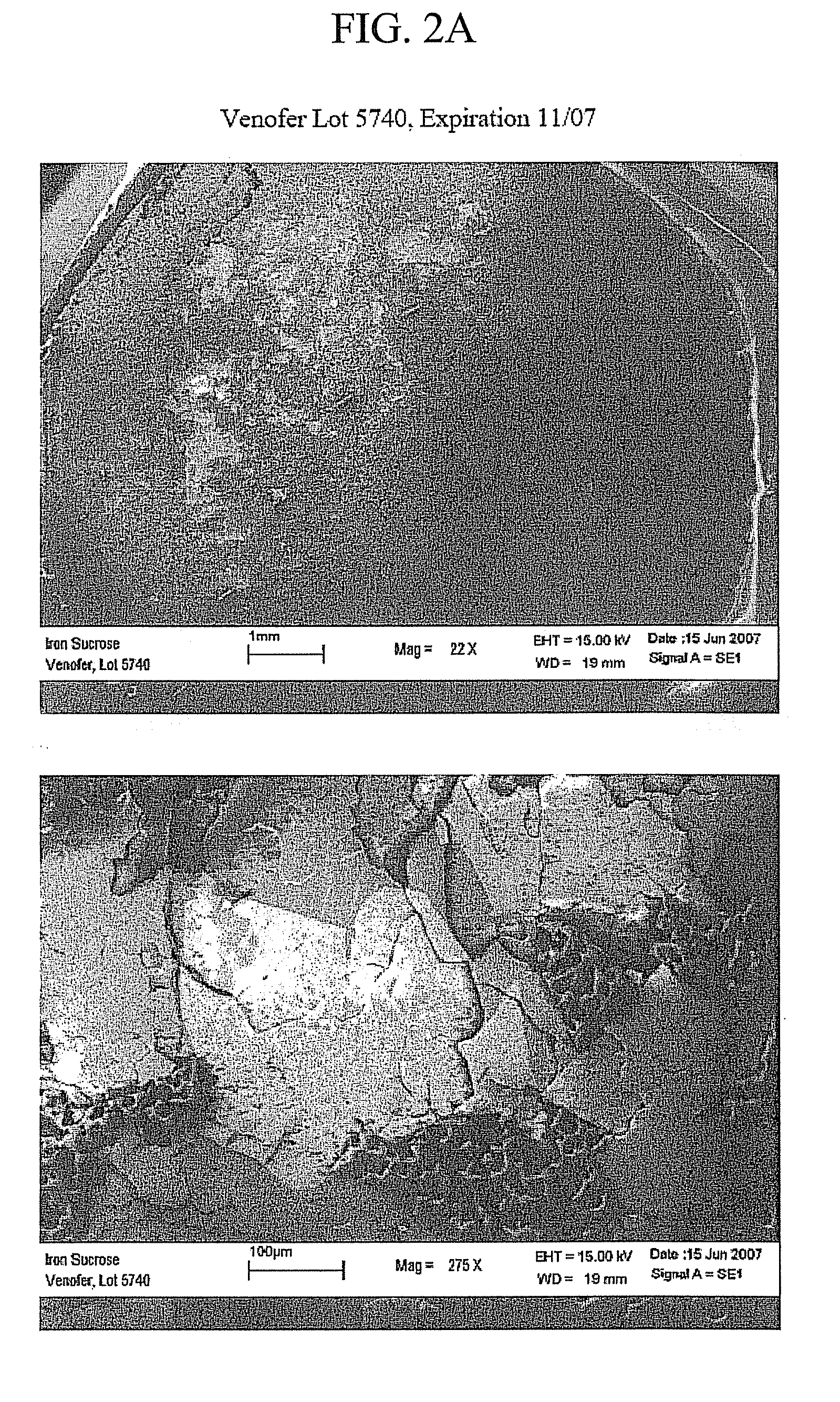
Packaged Iron Sucrose Products
a technology of iron sucrose and packaging, applied in the field of pharmaceutical products, can solve the problems of insufficient light obscuration technique to detect the delaminated particles in the iron sucrose formulation, the inability to easily recognize the presence of glass particulates as the result of delamination, and the greater cost of glass vials produced using this process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0048]A glass delamination study was performed under accelerated stability conditions. An iron sucrose solution (20 mg elemental iron and 300 mg sucrose per ml of water) at pH 11.0 was packaged in the containers along with a control wherein delamination is expected. Four different coated containers were evaluated to determine prevention of delamination under various packaging conditions. Molded glass vials (Wheaton Science Products, Milleville, N.J.), and glass tubing vials (Schott AG). were coated with silicone by rinsing the containers with the DOW CORNING® 365 Medical Fluid and baking the containers for a predetermined time and temperature. A third container was a CARPUJECT® syringe (Hospira, Inc., Lake Forest, Ill.). The syringe has a siliconized glass surface that is prepared by spraying the DOW CORNING® 365 medical fluid on the interior of the syringe and baking. The fourth container was a container of Schott TYPE 1 PLUS® tubing glass (Schott, AG), which is prepared with a pur...
example 2
[0051]In three additional studies, samples of Iron Sucrose Injection were prepared as described above and packaged in glass CARPUJECT® syringe containers that were coated with a silicone polymer as described in Example 1. The samples were subject to both accelerated and long term stability storage. Five units of each sample were collected at various time points and analyzed for glass flakes as described above. As shown in Table 2, some delamination was found in all samples stored at accelerated 40° C. storage after 6 months. However, as shown in Table 3, no delamination was found in all samples at 25° C. and 30° C. at 12 months of storage, and minimal delamination was found after 18 months of storage.
TABLE 2Time point / Iron Sucrose InjectionStorage ConditionSample ASample BSample CInitialNo delaminationNo delaminationNo delamination1M 40° C. / 75% RHNo delaminationNo delaminationNo delamination2M 40° C. / 75% RHNo delaminationNo delaminationNo delamination3M 40° C. / 75% RHNo delaminationN...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com