Modulating the Alternative Complement Pathway
a technology of alternative complement and pathway, applied in the field of modulating the alternative complement pathway, can solve the problems of insufficient local control of the alternative pathway by the target tissue, and the limited ability of circulating factor h to protect the surface of hypoxic tubular epithelial cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Factor H Purification, Analysis and Activities
Materials and Methods
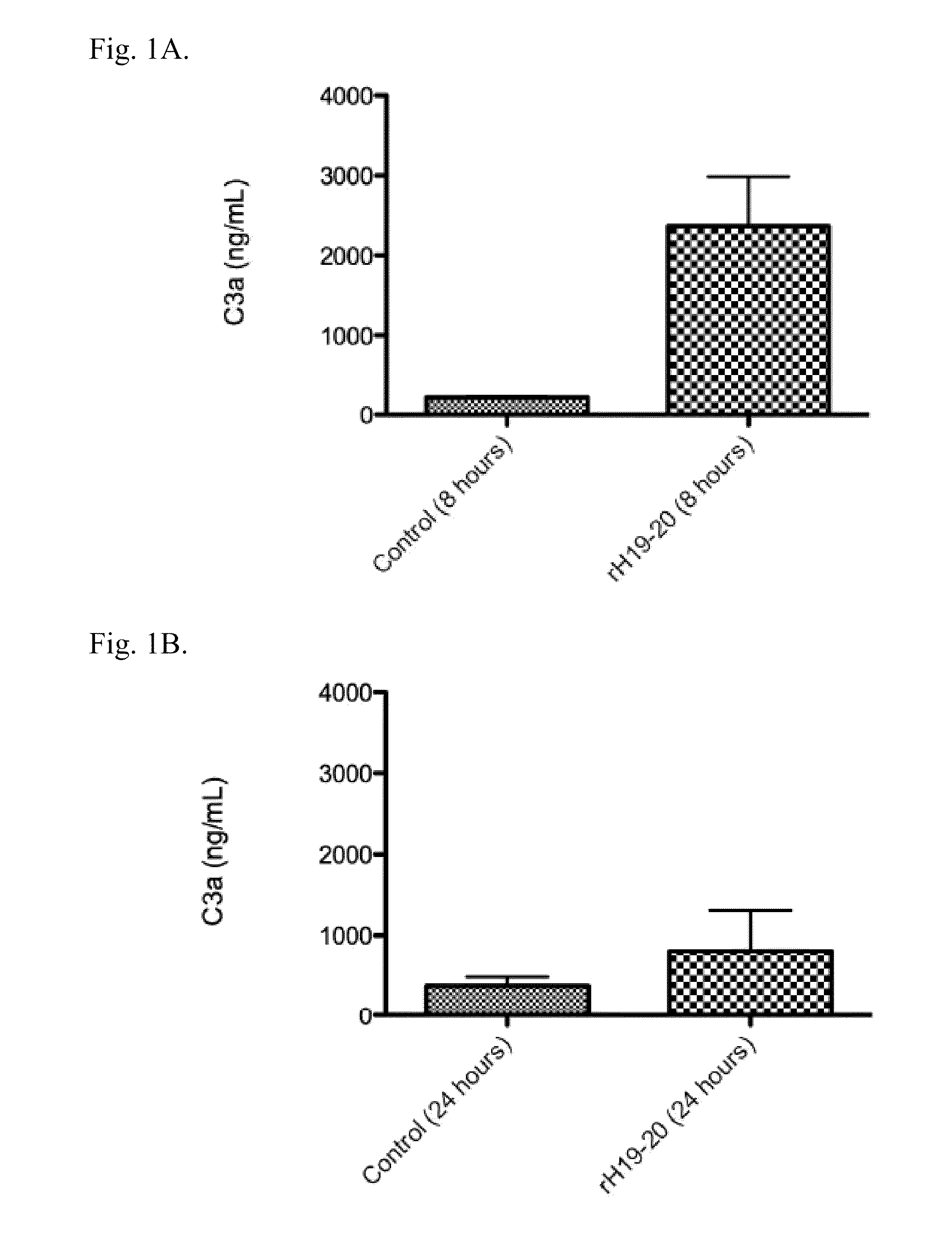
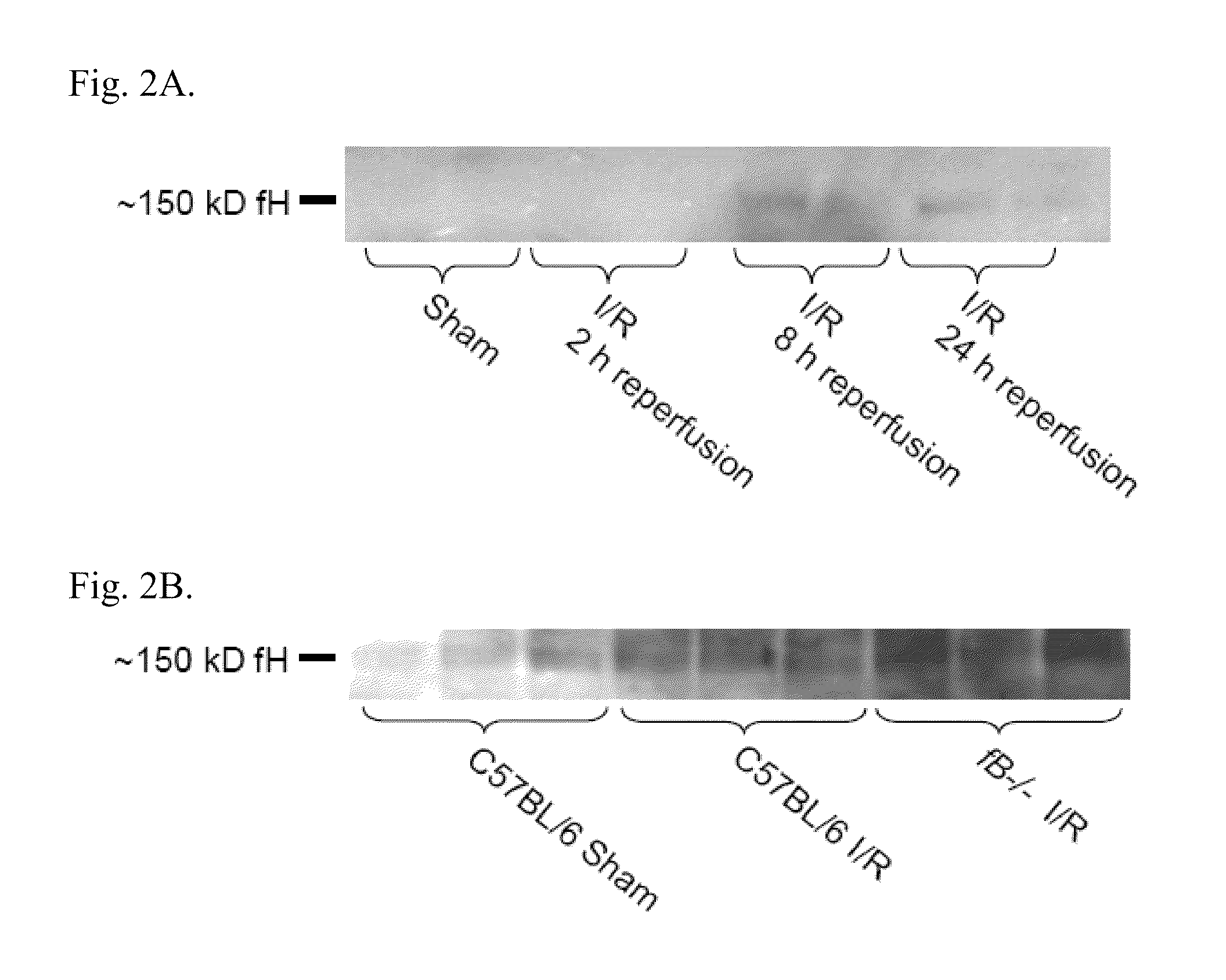
[0225]Purification of mouse factor H. An affinity column for factor H was made by ligating polyclonal goat antibody for human factor H (Quidel Corp., San Diego, Calif.) to Sepharose® derivatized with cyanogen bromide (CNBr) (Amersham Biosciences / GE Healthcare, Piscataway, N.J.) according to the manufacturer's instructions. Plasma was collected from C57BL / 6 mice and passed over the column. After washing the column with phosphate buffered saline (PBS), pH 7.4, the factor H was eluted with 5 M LiCl2. The salt solution was exchanged with PBS and the factor H was then passed through a Sephadex™ 26 / 60 Superdex™ 200 column (Amersham Biosciences / GE Healthcare, Piscataway, N.J.). The purity and identity of the isolated protein was verified by Coomassie staining and Western blot analysis by standard methods.
[0226]Western blot and Far Western blot analysis. Renal tissue was homogenized in radioimmunoprecipitation assay (RIPA) l...
example 2
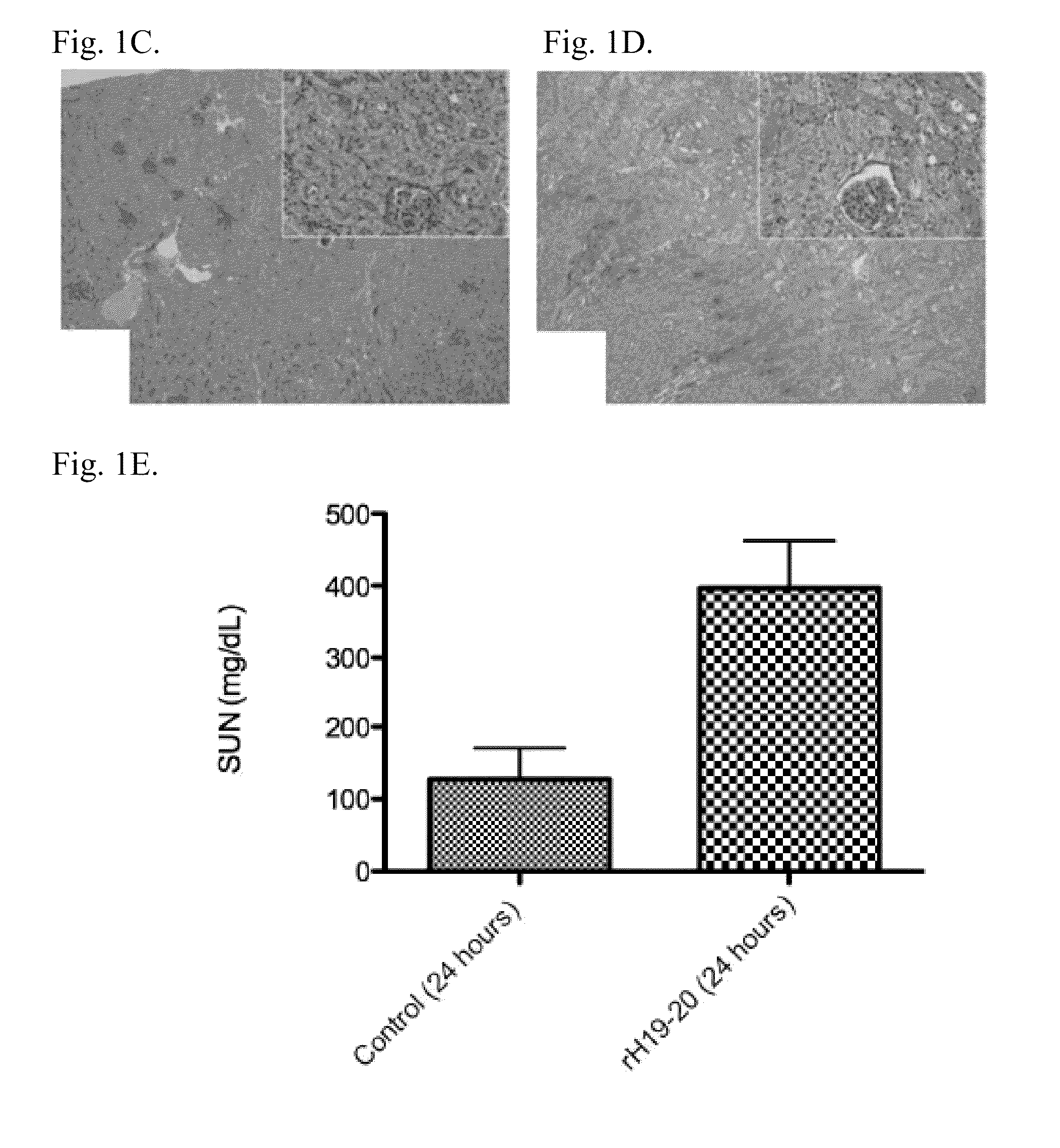
Effect of Annexin 2 During Renal Failure
Experimental
[0253]Renal ischemia / reperfusion protocol. Eight to ten week old male C57BL / 6J mice (Jackson Laboratories) mice weighing 20-25 g were anesthetized with 60 mg / kg ketamine plus 10 mg / kg xylazine (Vedco, Inc., St. Joseph, Mo.) injected intra-peritoneally. Mice were placed on a heating pad to maintain body temperature during surgery. Laparotomies were then performed and the renal pedicles were located and isolated by blunt dissection as known in the art. The pedicles were clamped with surgical clips (Miltex Instrument Company), and occlusion of blood flow was confirmed by visual inspection of the kidneys. The clamps were left in place for 24 minutes and then released. The kidneys were observed for approximately one minute to ensure blood re-flow, then fascia and skin were sutured with 4-0 silk (United States Surgical). Sham surgery was performed in an identical fashion, except that the renal pedicles were not clamped. The mice were vol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| full-length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| full-length factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com