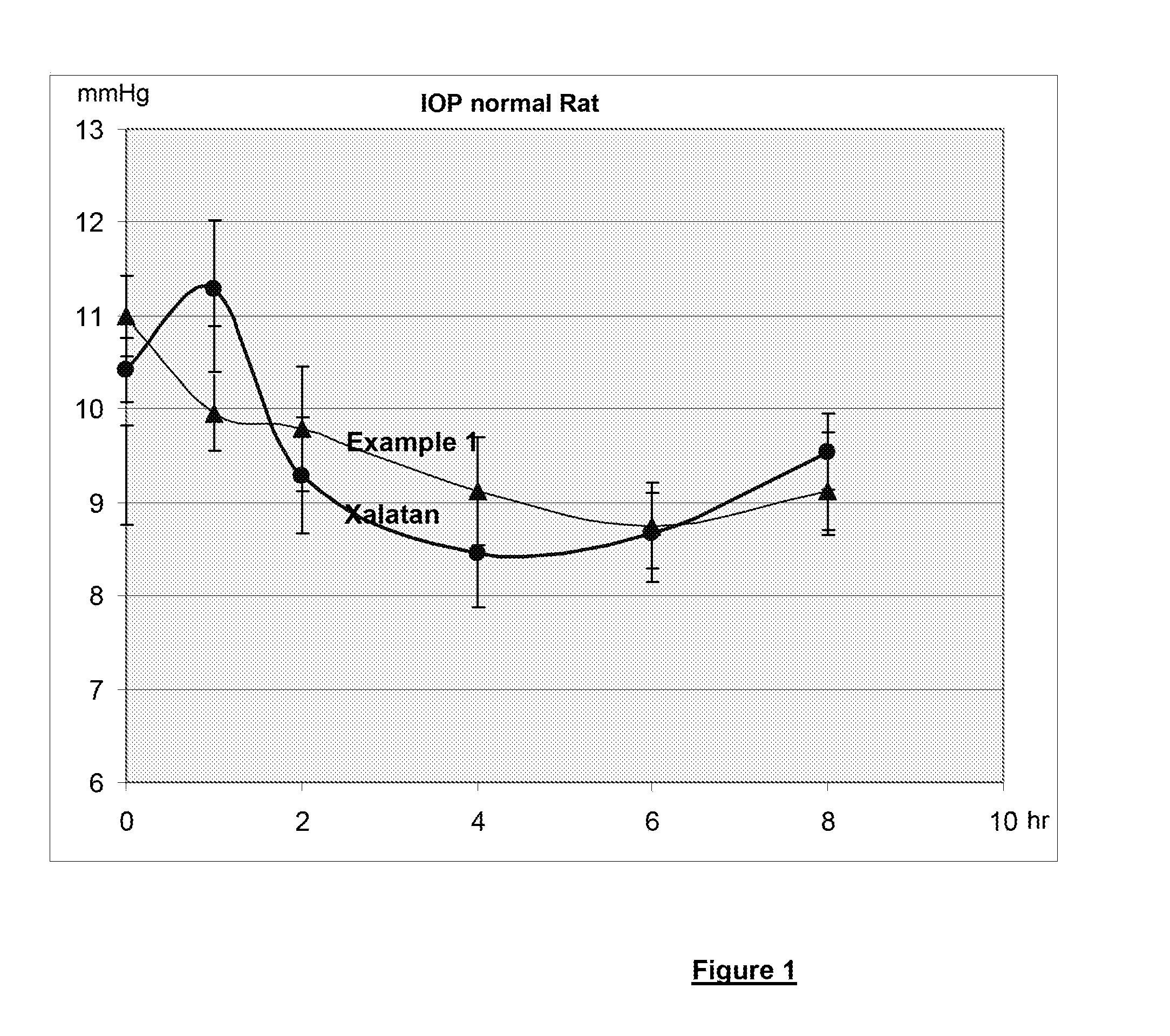
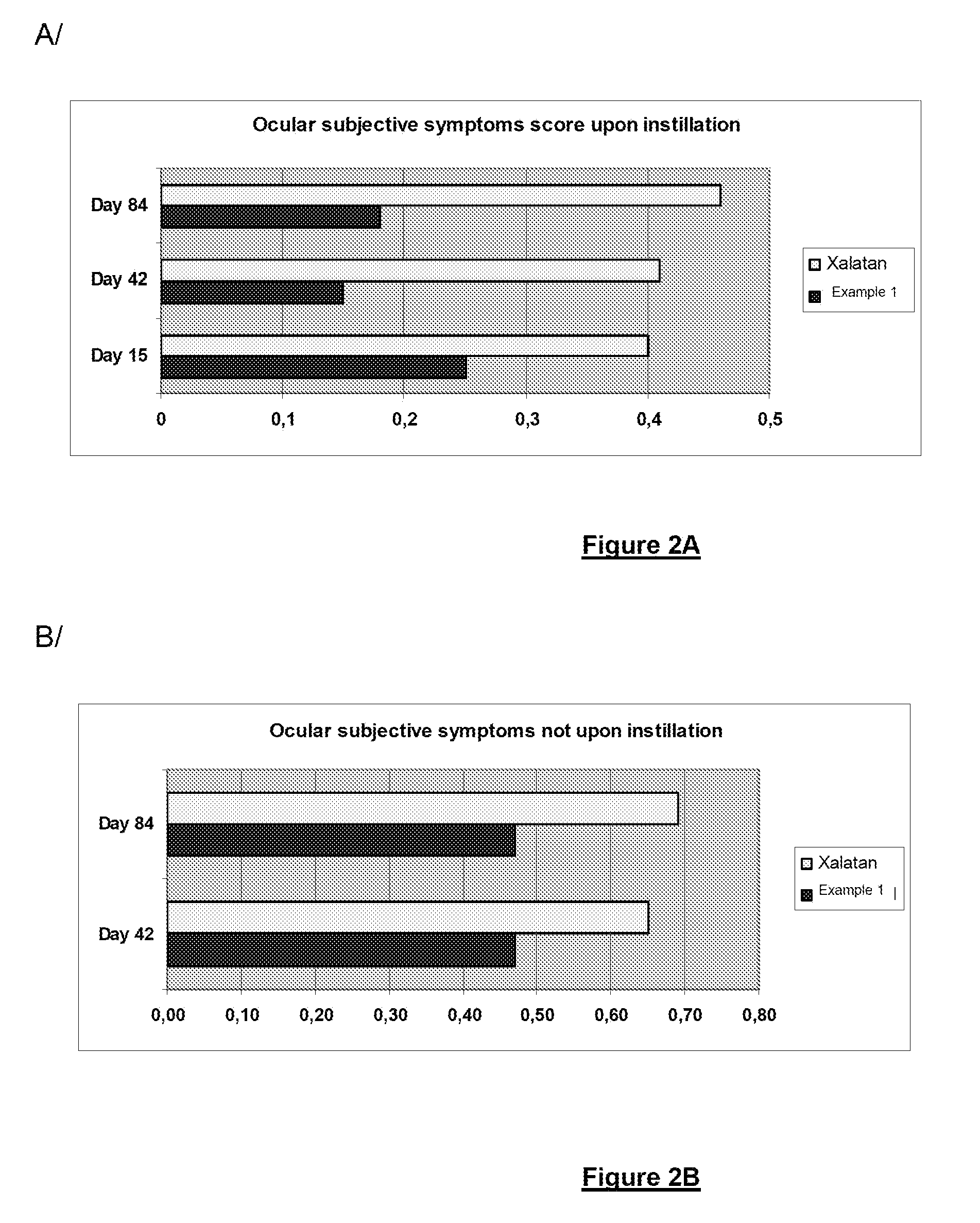
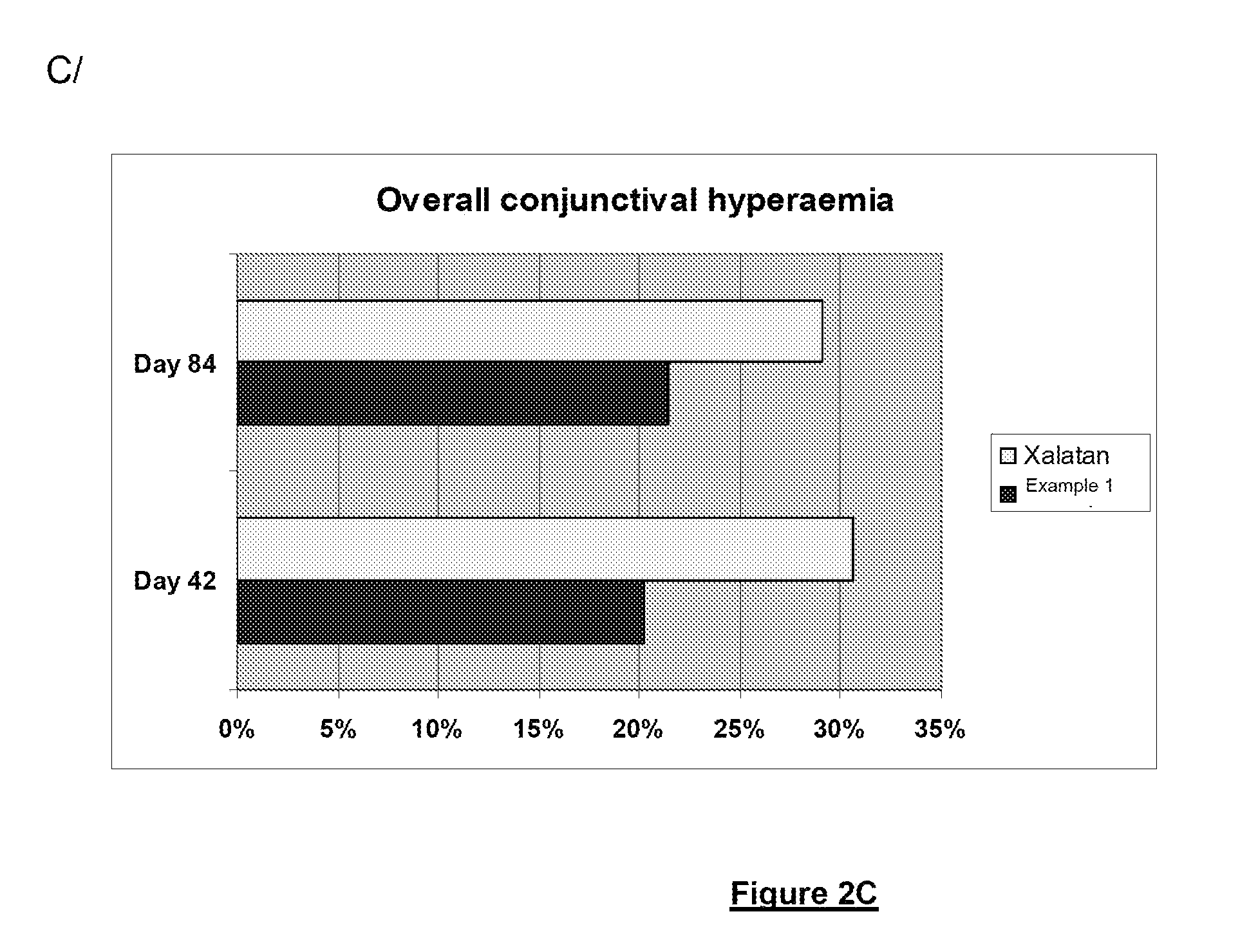
Polymeric delivery system for a nonviscous prostaglandin-based solution without preservatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1a / Example 1 Composition
[0055]
CENTESIMALFORMULAPRODUCTS(g / 100 ml)Carbomer / gelling agent0.10gSorbitol / isotonic agent3.50gPEG / co-gelling / co-solubilizing agent1.00gEDTA / Na+ ion source0.05g1N sodium hydroxide / Neutralizing agentqs pH = 7.0Latanoprost / Active ingredient0.005gMacrogol glycerol hydroxystearate 40 / solubilizing agent5.00gWFI-grade water / Vehicleqs 100ml
example 2
1b / Example 2 Composition
[0056]
CENTESIMALFORMULAPRODUCTS(g / 100 ml)Carbomer / gelling agent0.10gSorbitol / isotonic agent3.50gPVP / co-gelling / co-solubilizing agent2.00gSodium acetate / Na+ ion source0.8g1N sodium hydroxide / Neutralizing agentqs pH = 7.0Latanoprost / Active ingredient0.005gMacrogol glycerol hydroxystearate 40 / solubilizing agent5.00gWFI-grade water / Vehicleqs 100ml
1c / Example 3 Composition
[0057]
CENTESIMALFORMULAPRODUCTS(g / 100 ml)Carbomer / gelling agent0.15gSorbitol / isotonic agent2.25gPVA / co-gelling / co-solubilizing agent0.50gSodium chloride / Na+ ion source0.25g1N sodium hydroxide / Neutralizing agentqs pH = 7.0Travoprost / Active ingredient0.004gMacrogol 15 hydroxystearate / solubilizing agent0.50gWFI-grade water / Vehicleqs 100ml
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com