Production method for a metal nanostructure using an ionic liquid
a technology of metal nanostructure and ionic liquid, which is applied in the field of metal nanostructure production method, can solve the problems of difficult production of metal nanostructures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
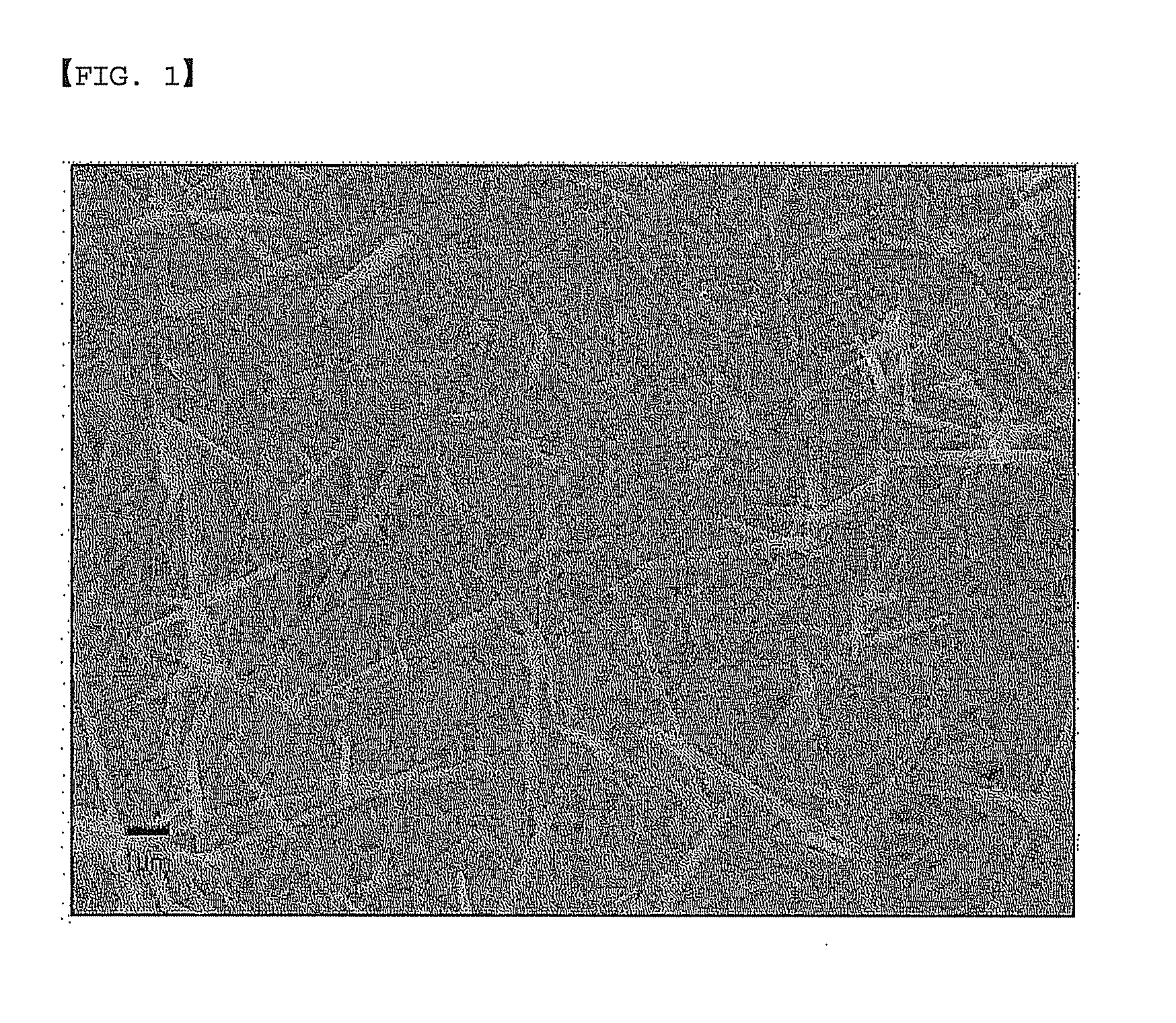
[0037]50 mL of a solution in which silver nitrate (AgNO3) is dissolved in ethyleneglycol to a concentration of 0.1 M was mixed with 50 mL of a solution in which 1-butyl-3-methylimidazolium methyl sulfate is dissolved in ethyleneglycol to a concentration of 0.15 M in a round-bottom flask to form a mixed solution. Subsequently, the mixed solution was stirred and reacted at 160° C. for 60 minutes, and was then cooled to room temperature. Subsequently, the cooled mixed solution was filtered with a filter having a pore size of 1 μm, and was then observed with an electron scanning microscope. As a result, it was found that metal nanowires were formed, as shown in FIG. 1. It was observed that the metal nanowires had a diameter of about 220 nm and a length of about 7 μm.
example 2
[0038]10 mL of a solution in which silver nitrate (AgNO3) is dissolved in 1,3-propyleneglycol to a concentration of 0.2 M was mixed with 10 mL of a solution in which 1-ethyl-3-methylimidazolium methyl sulfate is dissolved in 1,3-propyleneglycol to a concentration of 0.3 M in a round-bottom flask to form a mixed solution. Subsequently, the mixed solution was stirred and reacted at 100° C. for about 30 minutes, and was then cooled to room temperature. Subsequently, the cooled mixed solution was filtered with a filter having a pore size of 1 μm, and was then observed with an electron scanning microscope. As a result, it was found that metal nanowires having a diameter of about 180 nm and a length of about 10 μm were formed.
example 3
[0039]10 mL of a solution in which silver nitrate (AgNO3) is dissolved in 1,2-propyleneglycol to a concentration of 0.2 M was mixed with 10 mL of a solution in which 1-ethyl-3-methylimidazolium methyl sulfate is dissolved in 1,3-propyleneglycol to a concentration of 0.3 M in a round-bottom flask to form a first mixed solution, and then sodium dodecyl sulfate was added to the first mixed solution in an amount of 1% of the silver nitrate (AgNO3) to form a second mixed solution. Subsequently, the second mixed solution was stirred and reacted at 100° C. for about 30 minutes, and was then cooled to room temperature. Subsequently, the cooled second mixed solution was filtered with a filter having a pore size of 1 μm, and was then observed with an electron scanning microscope. As a result, it was found that metal nanowires having a diameter of about 80 nm and a length of about 10 μm were formed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com