Intragastric implants with duodenal anchors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
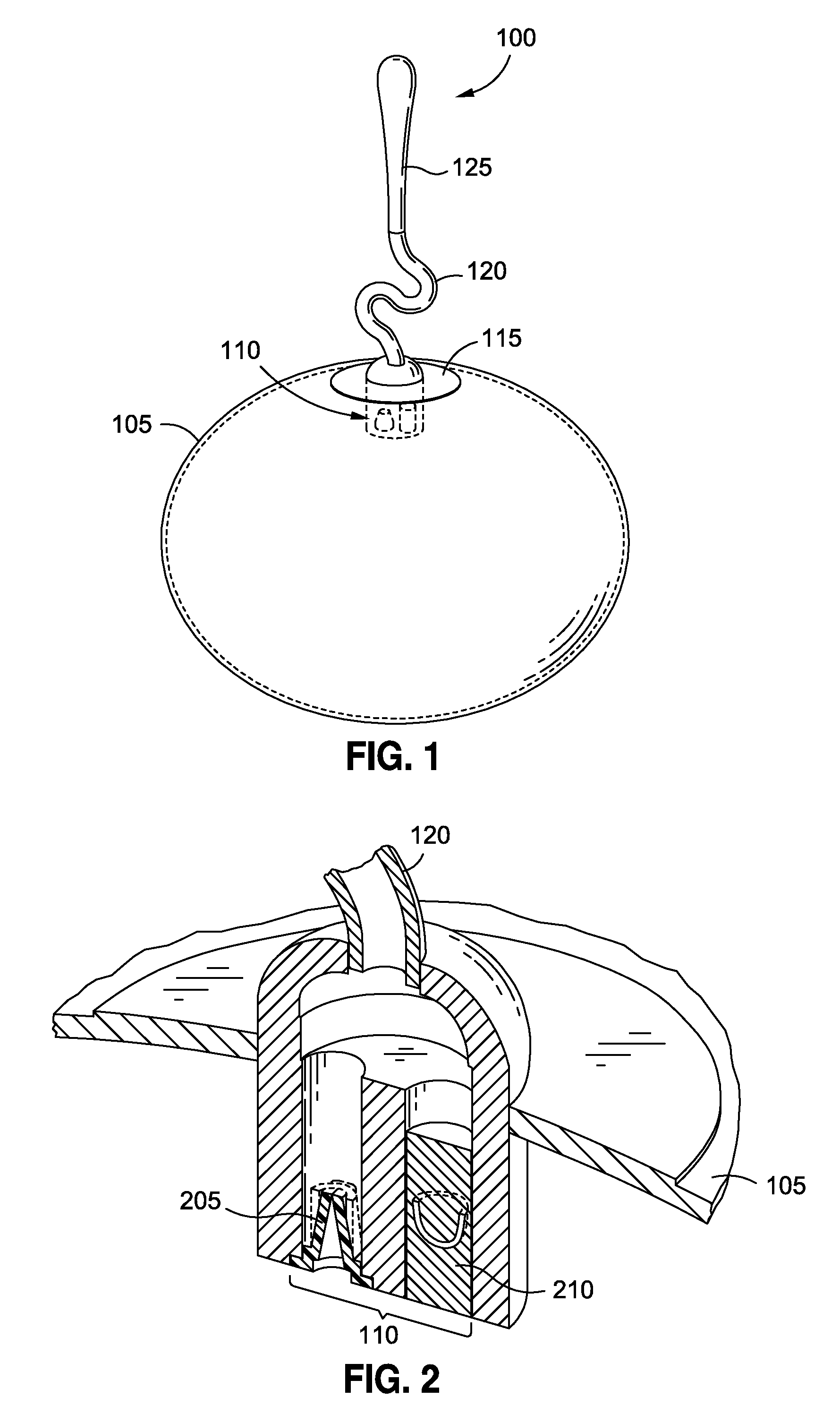
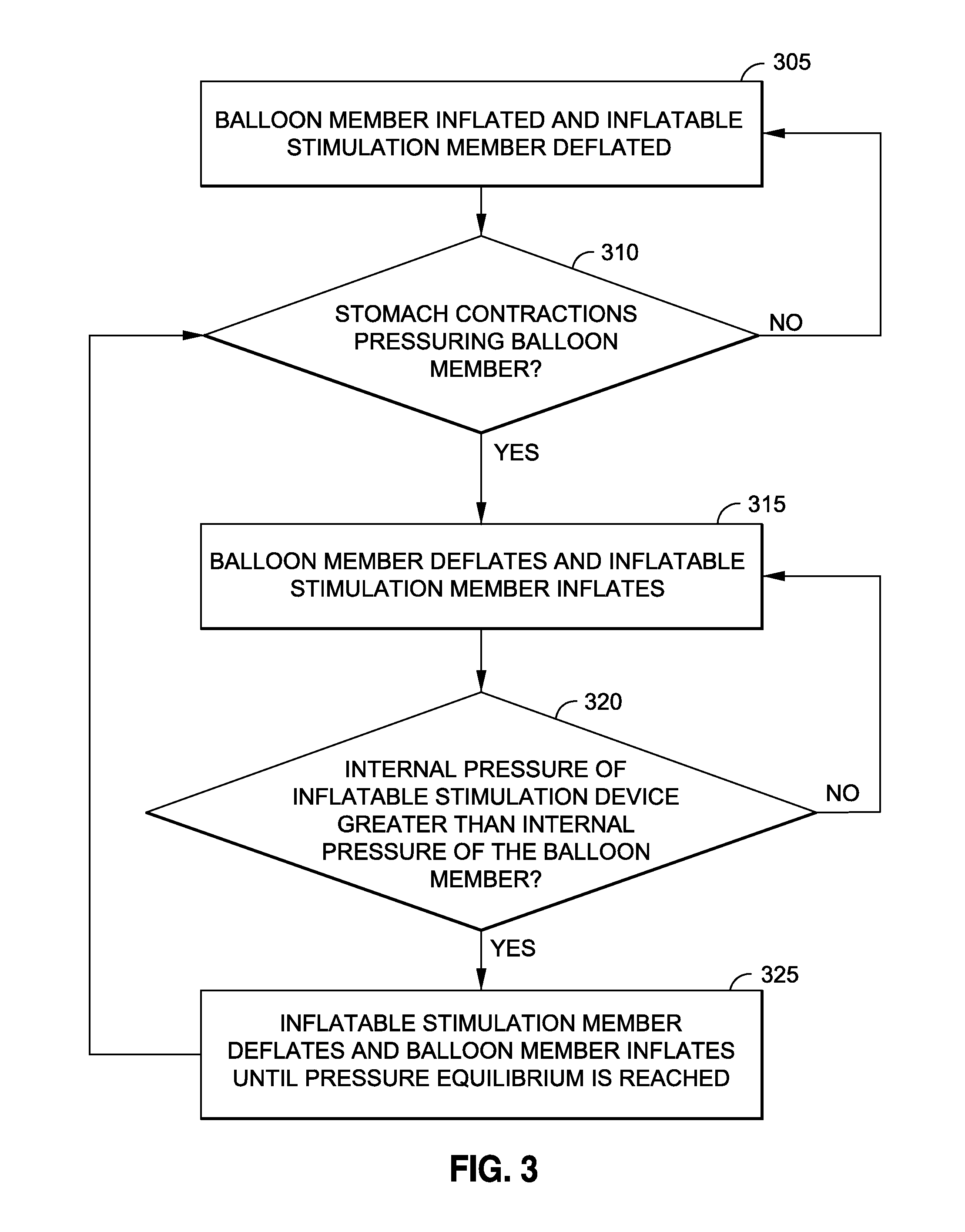
[0039]Persons skilled in the art will readily appreciate that various aspects of the disclosure may be realized by any number of methods and devices configured to perform the intended functions. Stated differently, other methods and devices may be incorporated herein to perform the intended functions. It should also be noted that the drawing FIGS. referred to herein are not all drawn to scale, but may be exaggerated to illustrate various aspects of the invention, and in that regard, the drawing FIGS. should not be construed as limiting. Finally, although the present disclosure may be described in connection with various medical principles and beliefs, the present disclosure should not be bound by theory.
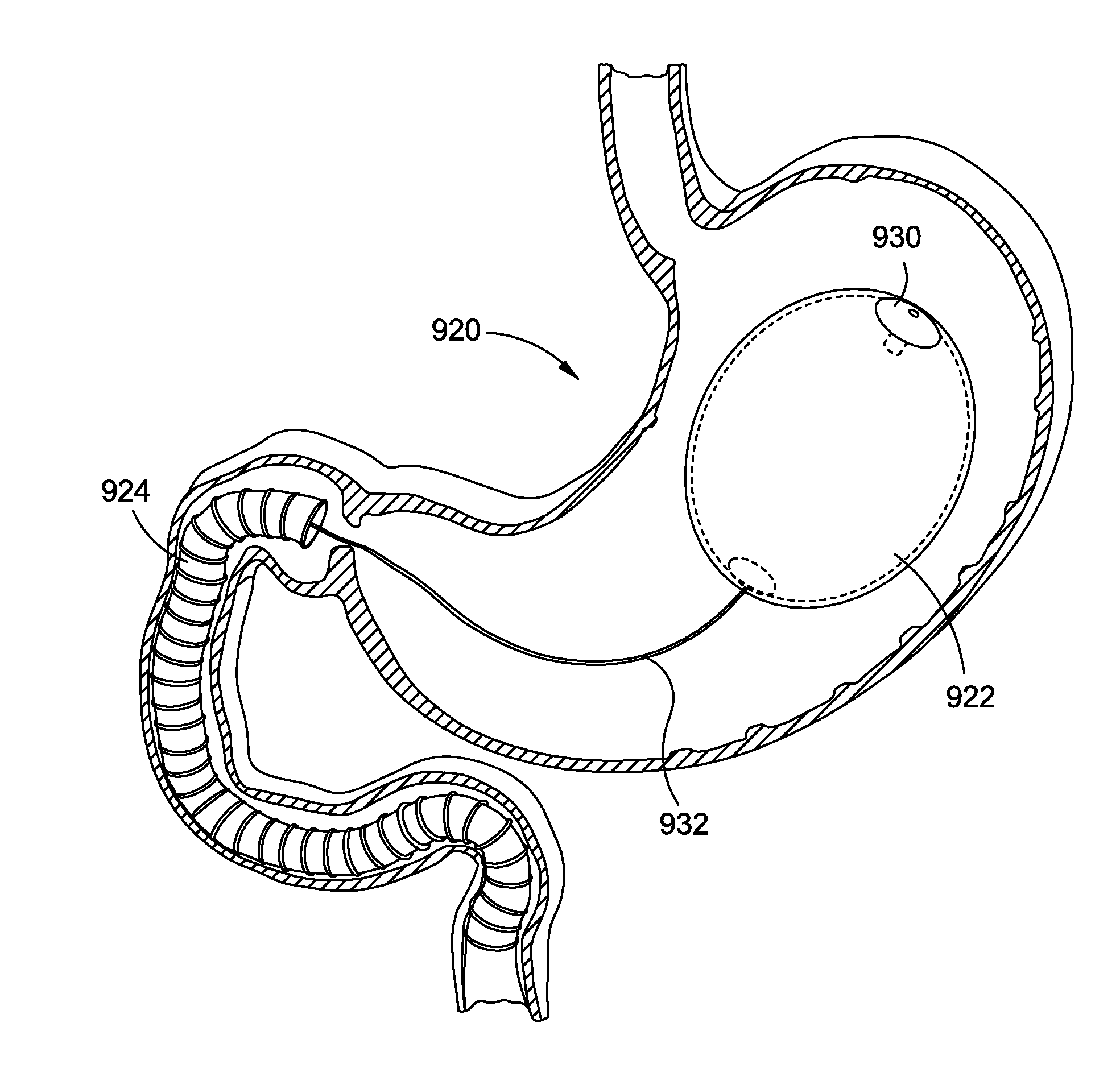
[0040]By way of example, the present disclosure will reference certain intragastric fluid transfer devices. Nevertheless, persons skilled in the art will readily appreciate that the present disclosure advantageously may be applied to one of the numerous varieties of intragastric flui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com