Composition for the treatment of cystic fibrosis
a cystic fibrosis and composition technology, applied in the field of composition for the treatment of cystic fibrosis, can solve the problems of inability to achieve the common cftr variant, and inability to treat the cystic fibrosis mechanism, so as to prevent or treat cystic fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
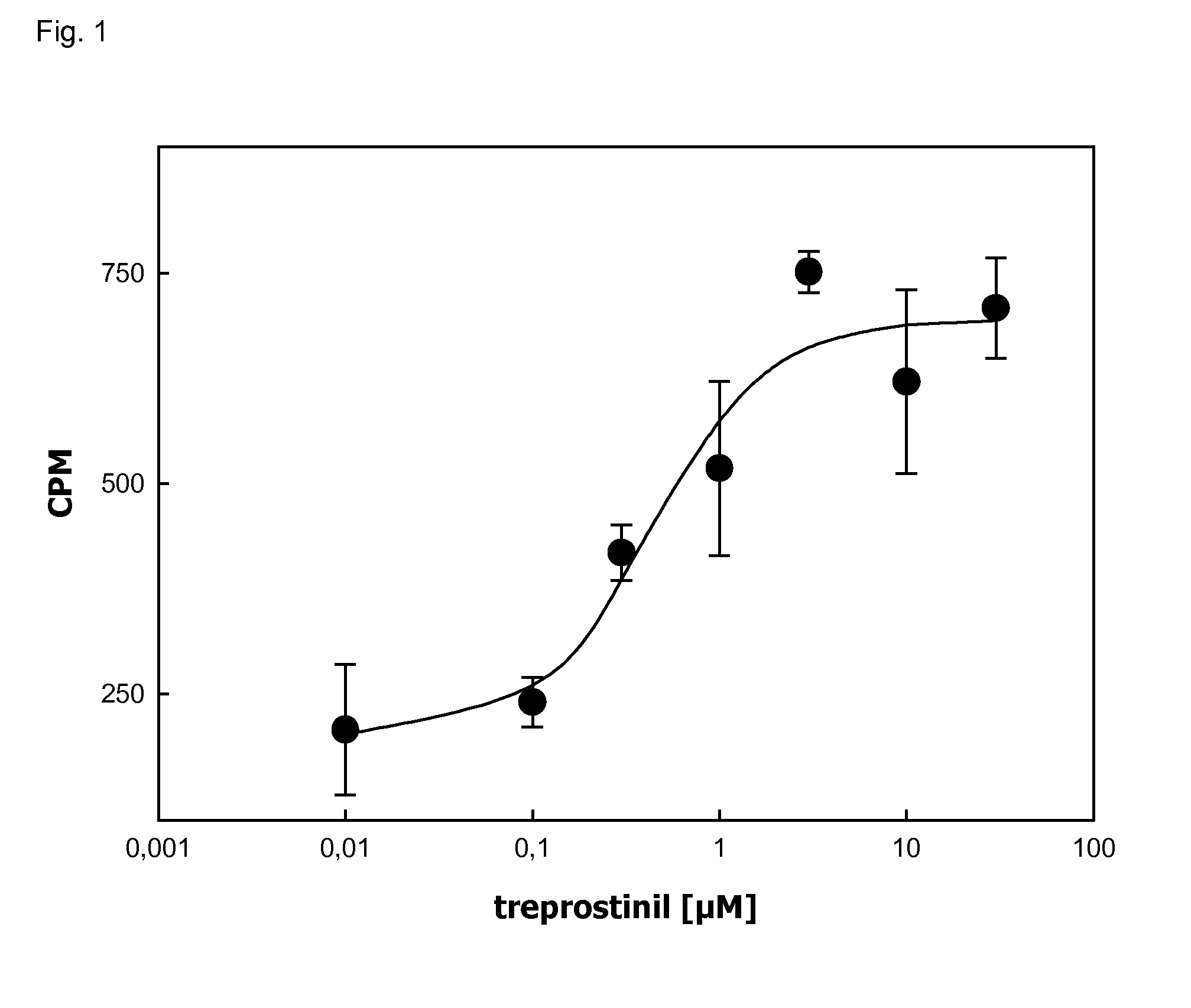
[0048]IB3-1 cells were plated on 6 well- plates (0.2*106 cells / well) in complete growth medium (LHC-8+5% FCS). The following day, the adenine nucleotide pool was metabolically labelled by incubation with [3H]adenine (1 μCi / well) in Dulbecco's Modified Eagle Medium (DMEM) containing adenosine deaminase (1 unit / ml) for 4 h. Cycic AMP formation was stimulated by 20 μM forskolin or the PGI2-analogue treprostinil (Remodulin®). The assay was performed in triplicates.
[0049]The formation of [3H]cAMP was determined by sequential chromatography on Dowex 50WX-4 and neutral alumina columns followed by liquid scintillation counting of the eluate.
[0050]Treprostinil caused a concentration-dependent accumulation of cAMP in IB3-1 cells (FIG. 1). Half-maximum stimulation was seen in the range of 0.3 to 1 μM.
example 2
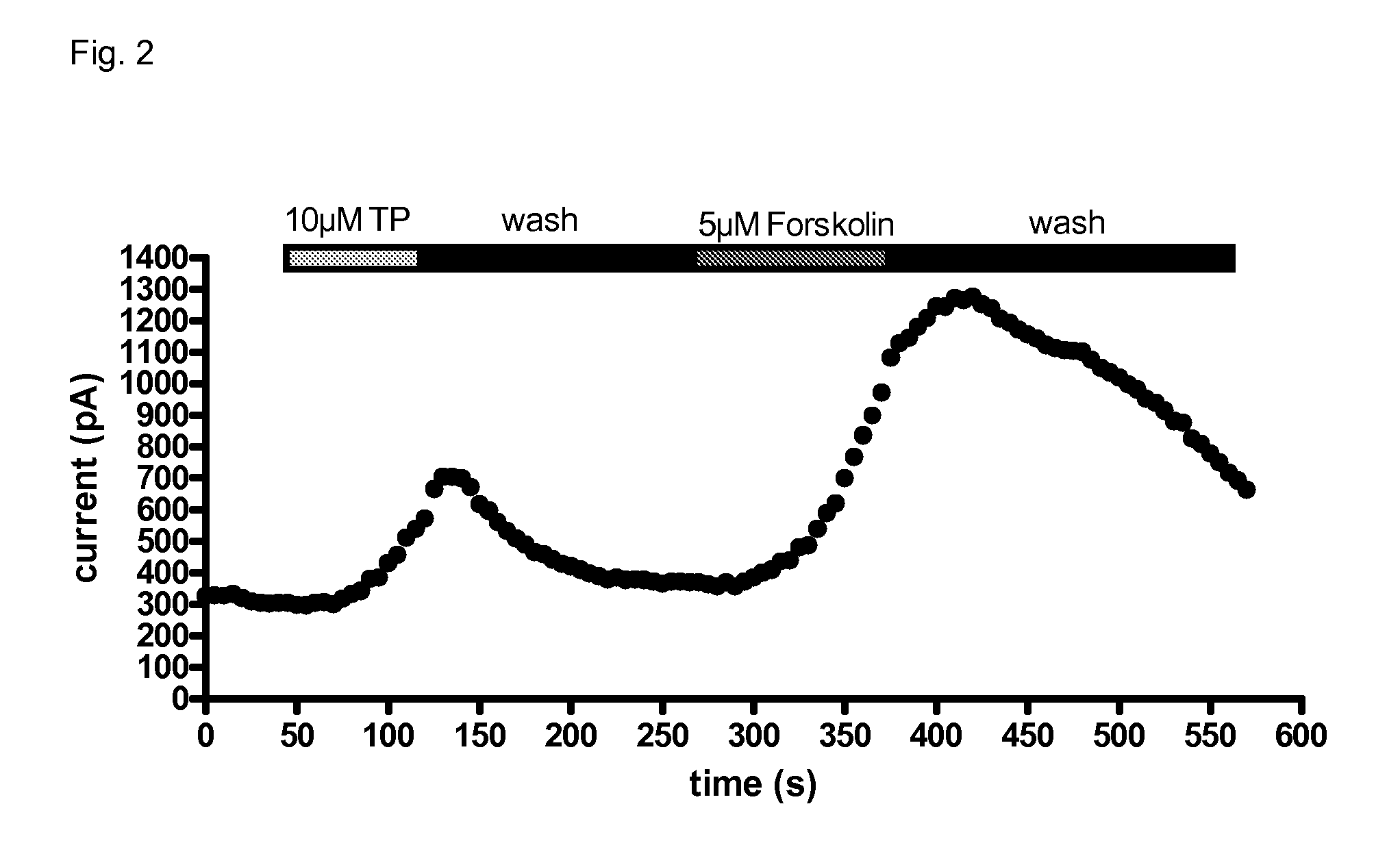
[0051]IB3-1 cells endogenously express only mutated CFTR-ΔF508, which is retained within the cells. Using appropriated manipulations (e.g., pharmacochaperones or low temperature incubations), it is possible to translocate the mutant CFTR-ΔF508 from the endoplasmic reticulum to the ER; when inserted at the cell surface, a Cl-conductance can be stimulated by elevating cAMP. The resulting Cl-conductance, however, is small. In order to unequivocally prove that the cAMP accumulation induced by Treprostinil translated into an activation of CFTR, we transiently expressed a GFP-tagged version of wild type CFTR (the GFP tag allowed for the identification of cells that expressed the protein at the cell surface). As can be seen from FIG. 2, Treprostinil caused a robust activation of the current induced by a depolarization from −40 mV holding potential to +60 mV. The maximum effect was delayed, i.e. it was only observed several s after wash-in of the compound. Likewise, there was also a hystere...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dimensionless property | aaaaa | aaaaa |
| Pharmaceutically acceptable | aaaaa | aaaaa |
| Body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com