Synthesis gas method and apparatus
a synthesis gas and gas technology, applied in the direction of products, organic chemistry, oxygen/ozone/oxide/hydroxide, etc., can solve the problems of degrading the reform catalyst used in connection, the conventional method of producing a synthesis gas such as the one discussed above, and the production of synthesis gas is not optimal, so as to reduce the slip of methane and increase the equilibrium temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
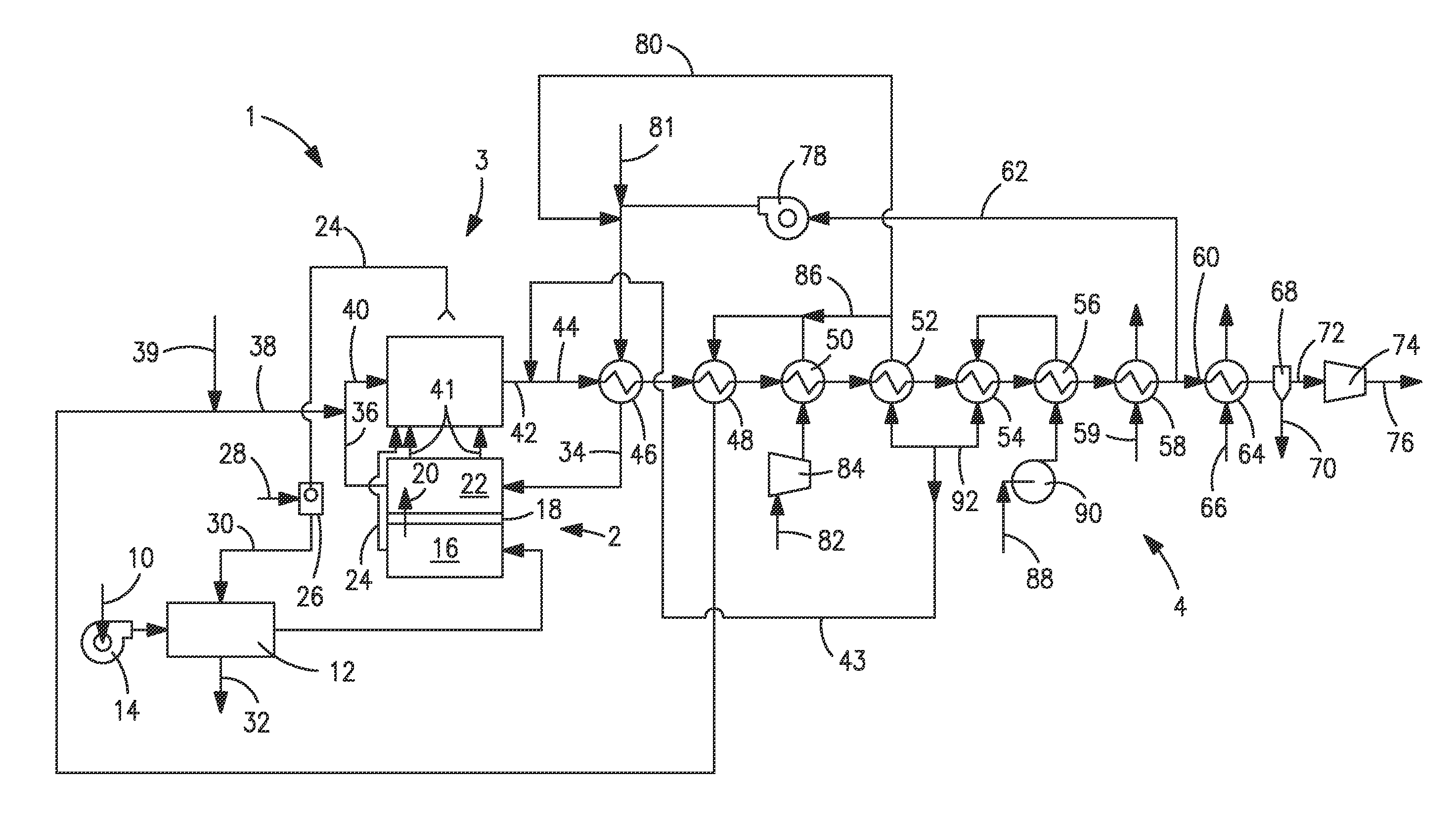
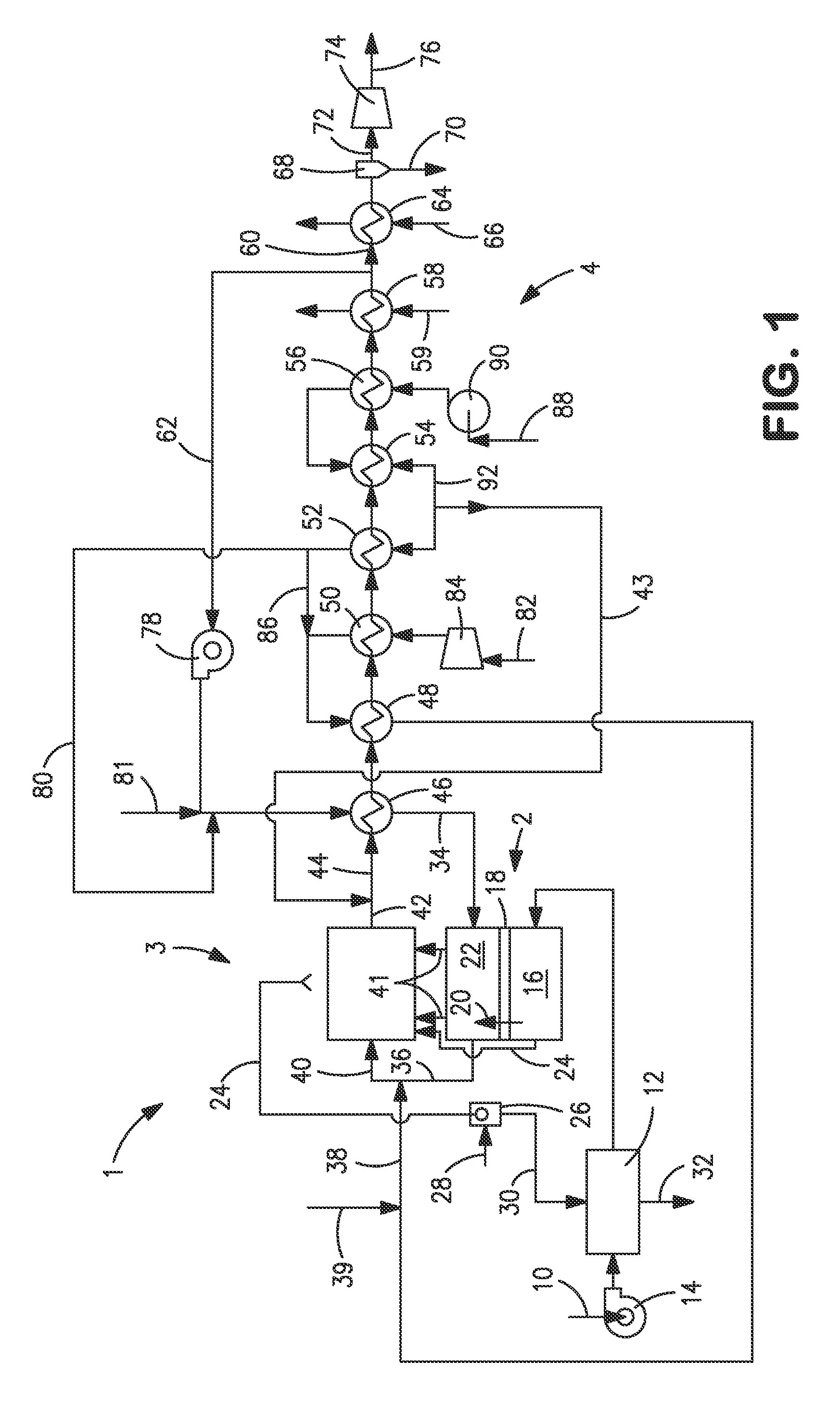
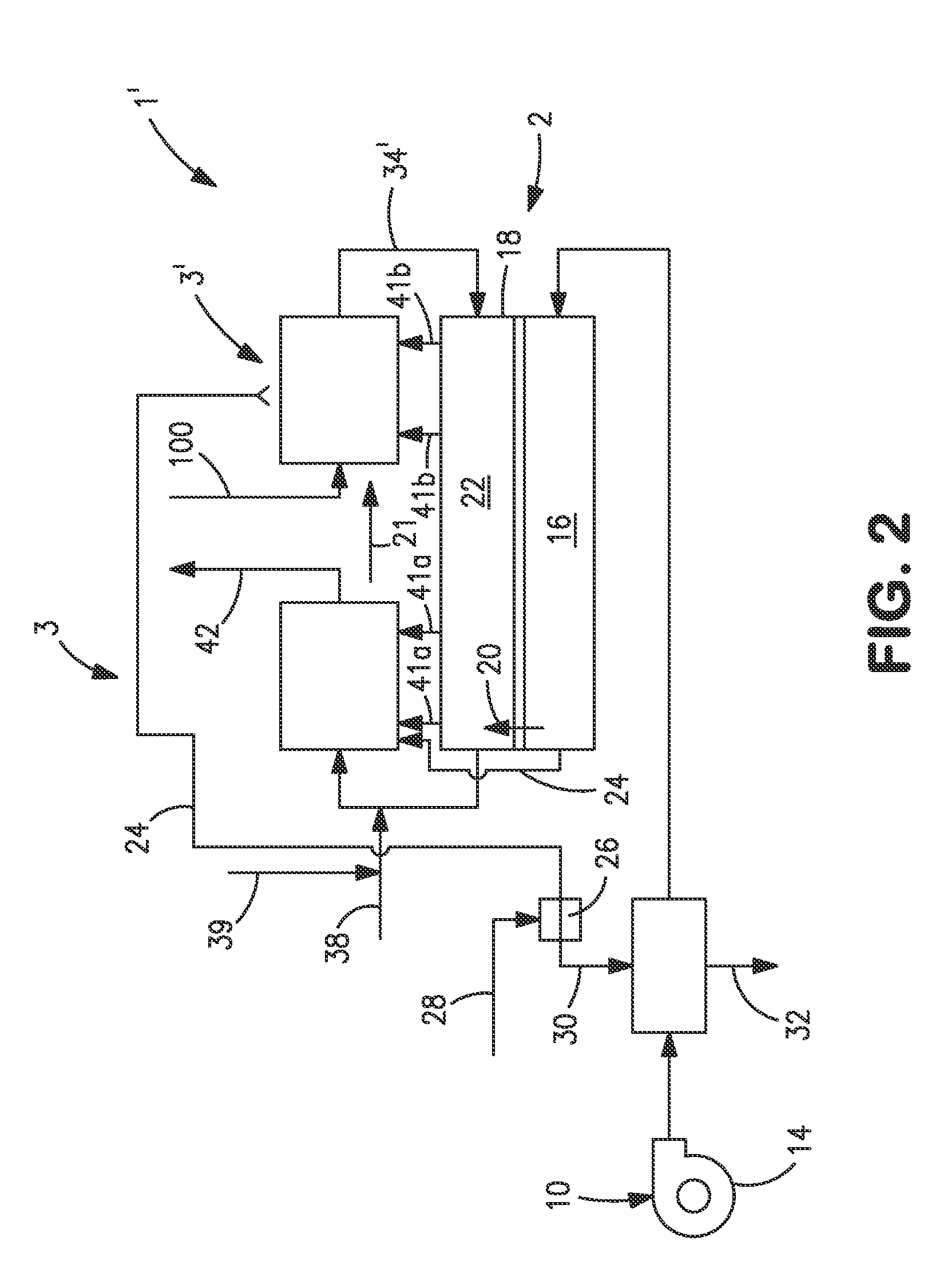
[0040]With reference to FIG. 1, an apparatus 1 is illustrated that is designed to produce a synthesis gas product through the steam methane reforming of hydrocarbons. Apparatus 1 includes one or more oxygen transport membrane elements of which oxygen transport membrane element 2 is illustrated. Oxygen transport membrane element 2 supplies heat by radiation and convective heat transfer to supply the endothermic heating requirements of a catalytic reactor 3 within which the hydrocarbons and steam are reacted to produce a synthesis gas. As well known in the art, at high temperatures, from 700 to 1100° C., steam will react with methane to yield a synthesis gas that contains hydrogen and carbon monoxide. Catalytic reactor 3, as would be known in the art, contains a catalyst, typically nickel, to promote such steam methane reforming reaction. Additionally, water-gas shift reactions occur in which the carbon monoxide will react with the steam to produce carbon dioxide and hydrogen. Althoug...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| pore diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com