Microorganisms for producing 1,4-butanediol and methods related thereto
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Biosynthesis of 4-Hydroxybutanoic Acid
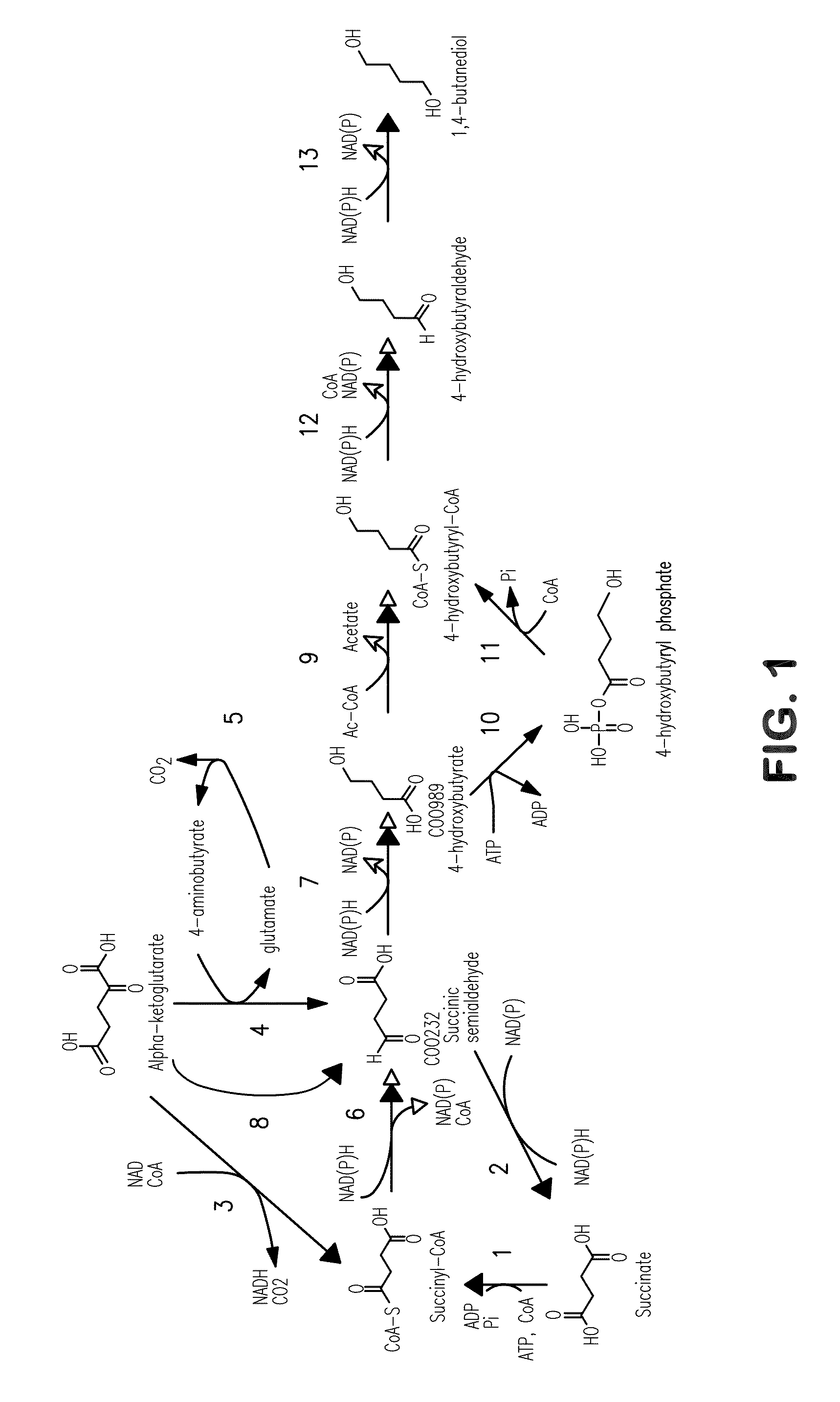
[0362]This example describes exemplary biochemical pathways for 4-HB production.
[0363]Previous reports of 4-HB synthesis in microbes have focused on this compound as an intermediate in production of the biodegradable plastic poly-hydroxyalkanoate (PHA) (U.S. Pat. No. 6,117,658). The use of 4-HB / 3-HB copolymers over poly-3-hydroxybutyrate polymer (PHB) can result in plastic that is less brittle (Saito and Doi, Intl. J. Biol. Macromol. 16:99-104 (1994)). The production of monomeric 4-HB described herein is a fundamentally distinct process for several reasons: (1) the product is secreted, as opposed to PHA which is produced intracellularly and remains in the cell; (2) for organisms that produce hydroxybutanoate polymers, free 4-HB is not produced, but rather the Coenzyme A derivative is used by the polyhydroxyalkanoate synthase; (3) in the case of the polymer, formation of the granular product changes thermodynamics; and (4) extracellular pH is not...
example ii
Biosynthesis of 1,4-Butanediol from Succinate and Alpha-Ketoglutarate
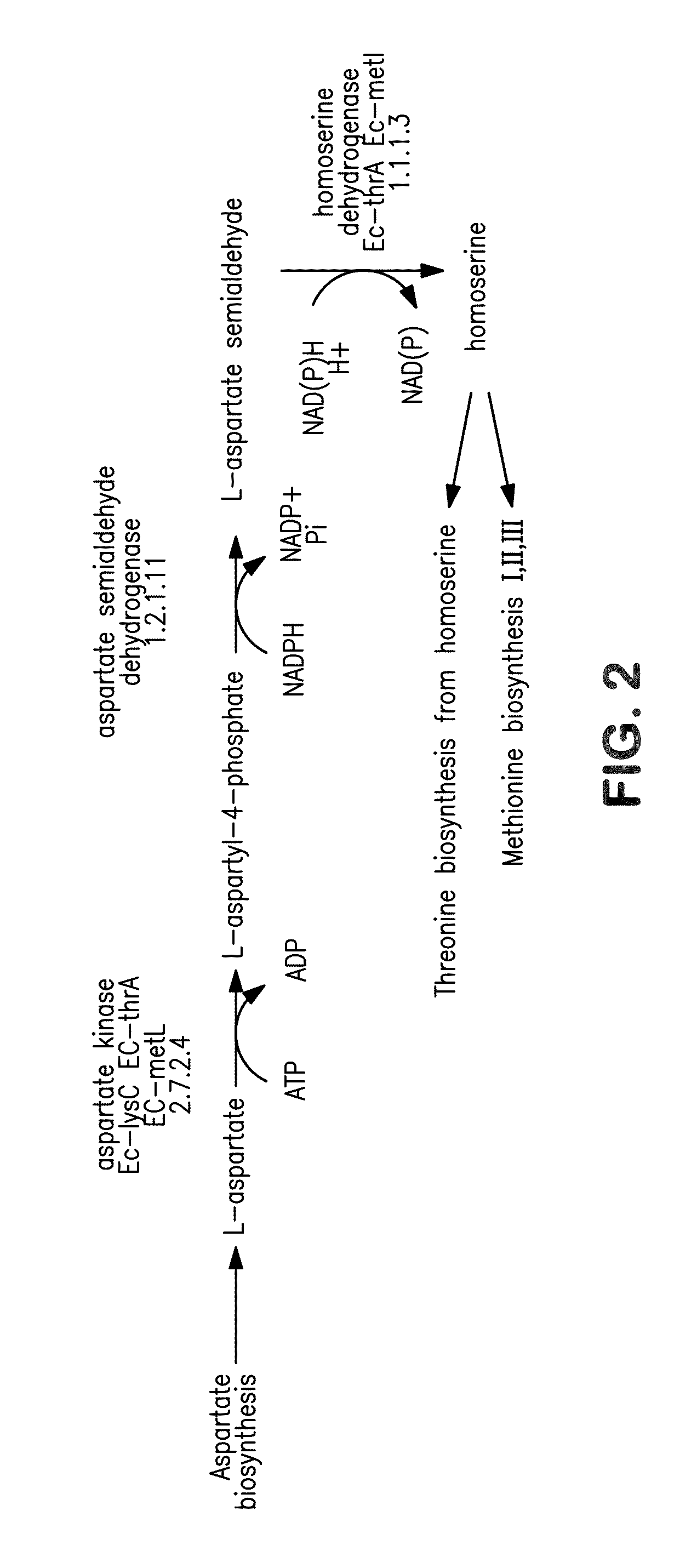
[0369]This example illustrates the construction and biosynthetic production of 4-HB and BDO from microbial organisms. Pathways for 4-HB and BDO are disclosed herein.
[0370]There are several alternative enzymes that can be utilized in the pathway described above. The native or endogenous enzyme for conversion of succinate to succinyl-CoA (Step 1 in FIG. 1) can be replaced by a CoA transferase such as that encoded by the cat1 gene C. kluyveri (Sohling and Gottschalk, Eur. J Biochem. 212:121-127 (1993)), which functions in a similar manner to Step 9. However, the production of acetate by this enzyme may not be optimal, as it might be secreted rather than being converted back to acetyl-CoA. In this respect, it also can be beneficial to eliminate acetate formation in Step 9. As one alternative to this CoA transferase, a mechanism can be employed in which the 4-HB is first phosphorylated by ATP and then converted to the C...
example iii
Biosynthesis of 4-Hydroxybutanoic Acid, γ-Butyrolactone and 1,4-Butanediol
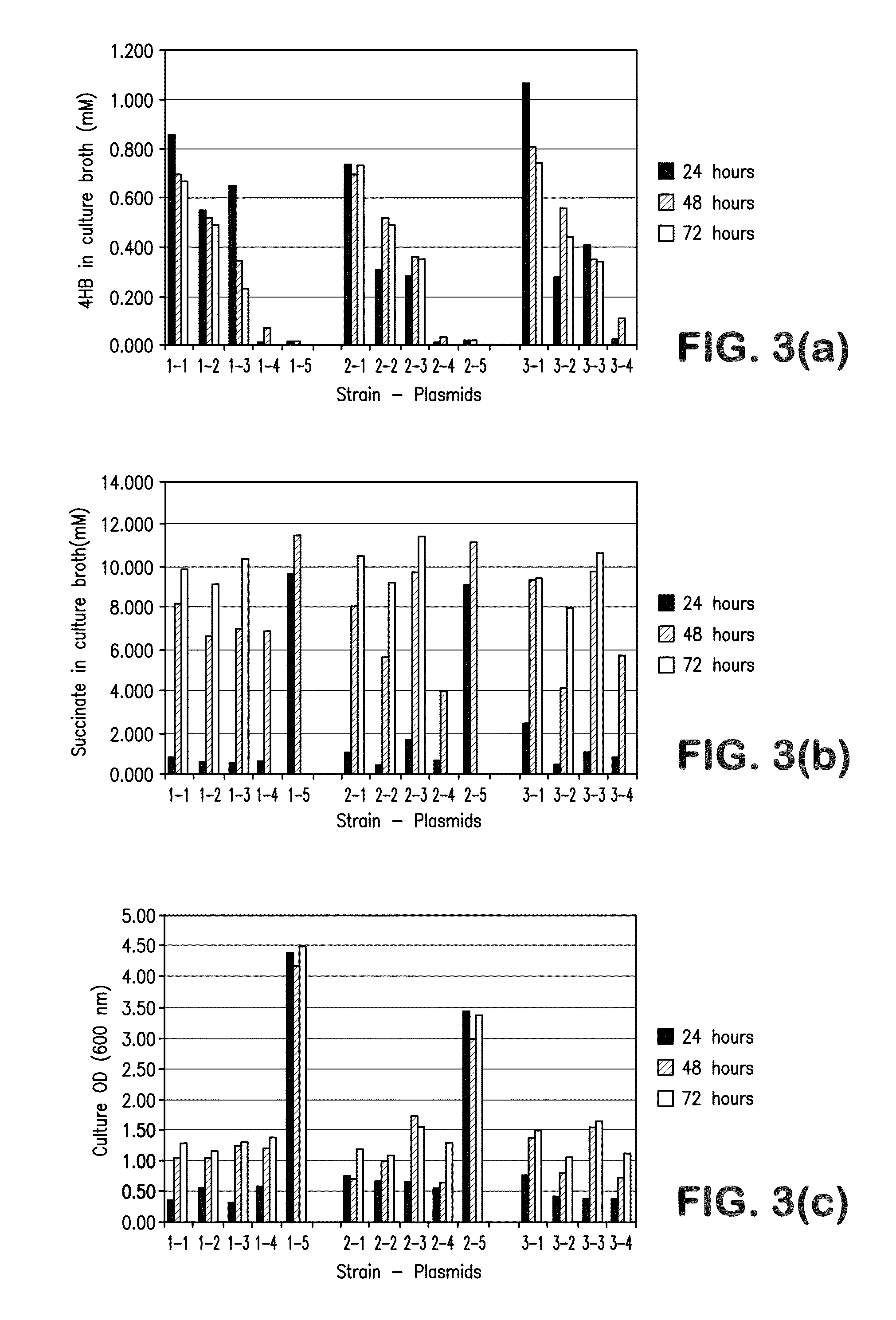
[0402]This Example describes the biosynthetic production of 4-hydroxybutanoic acid, γ-butyrolactone and 1,4-butanediol using fermentation and other bioprocesses.
[0403]Methods for the integration of the 4-HB fermentation step into a complete process for the production of purified GBL, 1,4-butanediol (BDO) and tetrahydrofuran (THF) are described below. Since 4-HB and GBL are in equilibrium, the fermentation broth will contain both compounds. At low pH this equilibrium is shifted to favor GBL. Therefore, the fermentation can operate at pH 7.5 or less, generally pH 5.5 or less. After removal of biomass, the product stream enters into a separation step in which GBL is removed and the remaining stream enriched in 4-HB is recycled. Finally, GBL is distilled to remove any impurities. The process operates in one of three ways: 1) fed-batch fermentation and batch separation; 2) fed-batch fermentation and continuous sepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com