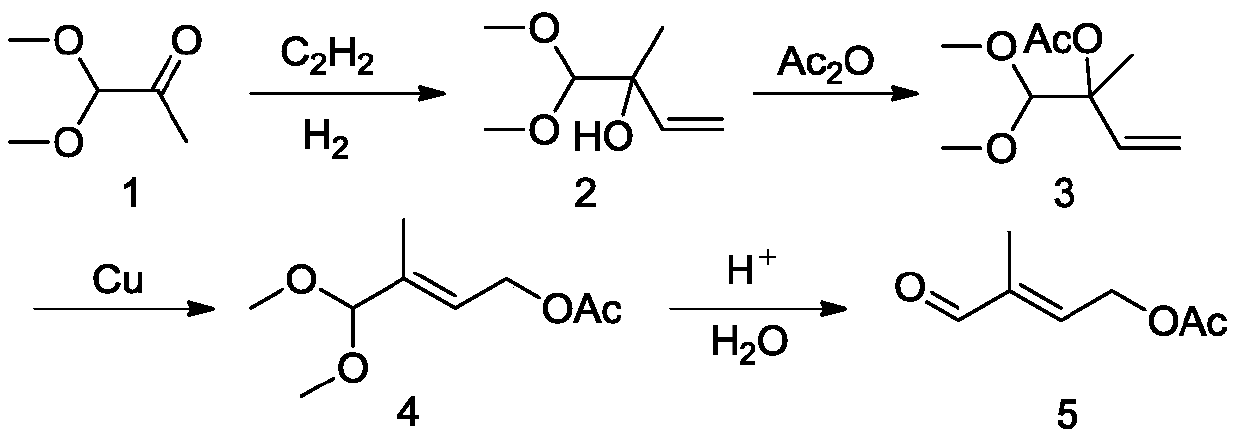
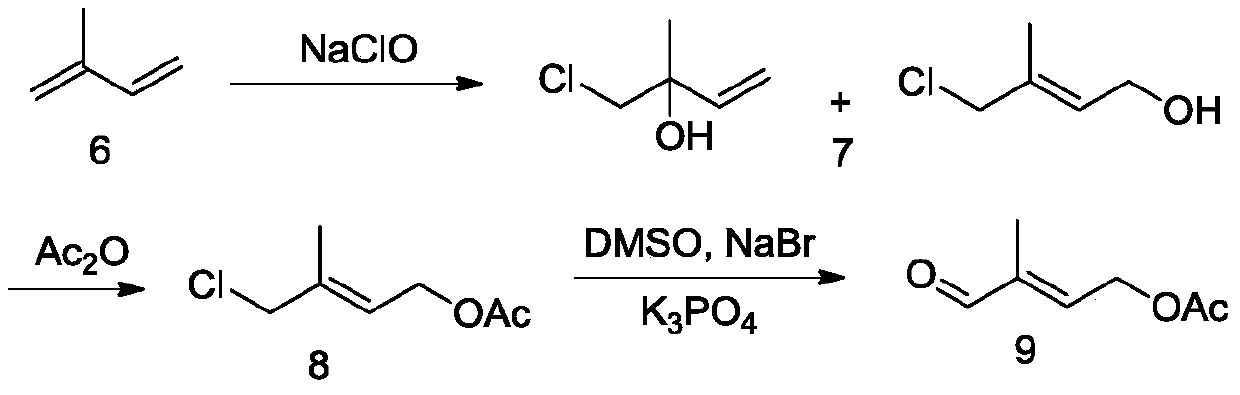
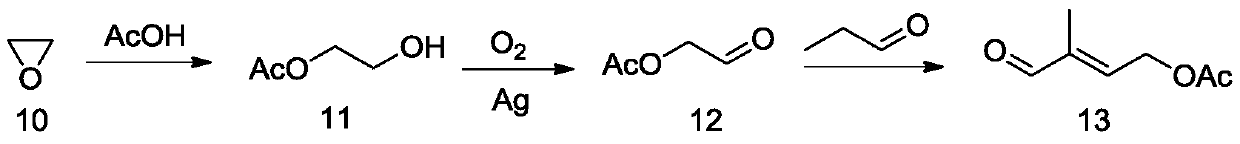
Method for preparing 4-acetoxy-2-methyl-2-crotonaldehyde
A technology of butyraldehyde and hydroxybutyraldehyde, which is applied in the field of preparation of 4-acetoxy-2-methyl-2-butenal, can solve problems such as high price, achieve less waste water, be environmentally friendly, and avoid phosphine Effects of the use of catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Preparation of 2-methylene-4-hydroxybutyraldehyde:
[0051] Add 1L tetrahydrofuran into a 3L glass three-neck flask, turn on the mechanical stirring, set the speed at 800rpm, cool down to -30°C, then add 2mol n-butyllithium and 2.5mol diisopropylamine to it, react for 0.5h, add 4-hydroxybutyl Add 1 mol of aldehyde, stir for 0.5 h, add 1 mol of (N,N-dimethyl)methylene ammonium iodide, and react at room temperature for 8 h. After the reaction is over, add 0.5 L of water to the system, extract three times with dichloromethane, 0.5 L each time, combine the organic phases, reclaim the dichloromethane under reduced pressure, and rectify the mother liquor under the condition of 1kPa with a packed column with a theoretical plate number of 20. The reaction solution after removing the solvent, the reflux ratio is 2:1, collect the distillate at 38-39°C at the top of the tower to obtain 2-methylene-4-hydroxybutyraldehyde, and analyze 2-methylene-4-hydroxybutyraldehyde by gas ch...
Embodiment 2
[0057] (1) Preparation of 2-methylene-4-hydroxybutyraldehyde:
[0058] Add 1L of toluene to a 3L glass three-neck flask, turn on mechanical stirring, set the speed at 800rpm, cool down to -40°C, then add 1.5mol of n-butyllithium and 2mol of diisopropylamine to it, react for 1h, then add 4-hydroxybutyraldehyde 1mol, stirred for 1h, added (N,N-dimethyl) methylene ammonium iodide 1mol, then reacted at room temperature for 6h. After the reaction is over, add 0.5 L of water to the system, extract three times with dichloromethane, 0.5 L each time, combine the organic phases, reclaim the dichloromethane under reduced pressure, and rectify the mother liquor under the condition of 1kPa with a packed column with a theoretical plate number of 20. The reaction solution after removing the solvent, the reflux ratio is 2:1, collect the distillate at 38-39°C at the top of the tower to obtain 2-methylene-4-hydroxybutyraldehyde, and analyze 2-methylene-4-hydroxybutyraldehyde by gas chromatograp...
Embodiment 3
[0064] (1) Preparation of 2-methylene-4-hydroxybutyraldehyde:
[0065] Add 1.5L tetrahydrofuran to a 3L flask, start stirring, set the speed at 800rpm, cool down to -10°C, then add 3mol n-butyl lithium and 2.5mol diisopropylamine to it, react for 2.5h, then add 1mol of 4-hydroxybutyraldehyde , stirred for 2.5h, added (N,N-dimethyl) methylene ammonium iodide 1.5mol, and then reacted at room temperature for 12h. After the reaction is over, add 0.5 L of water to the system, extract three times with dichloromethane, 0.5 L each time, combine the organic phases, reclaim the dichloromethane under reduced pressure, and rectify the mother liquor under the condition of 1kPa with a packed column with a theoretical plate number of 20. The reaction solution after removing the solvent, the reflux ratio is 2:1, collect the distillate at 38-39°C at the top of the tower to obtain 2-methylene-4-hydroxybutyraldehyde, and analyze 2-methylene-4-hydroxybutyraldehyde by gas chromatography The aldeh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com