Inhibitors for treating and preventing heart failure in felines
a technology of inhibitors and heart failure, applied in the field of veterinary medicine, can solve the problems of heart failure, undesired side effects, and complicating the interpretation of the effect of heart rate, so as to improve the quality of life and reduce the risk of death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation of a Compound for the Treatment of a Heart Disease
[0079]This example provides the formulation of compound (+)-3-[(N-(2-(3,4-dimethoxy-phenyl)ethyl)-piperidin-3-(S)-yl)-methyl]-(7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepin-2-on hydrochloride in liquid form. For the production of multi-layered particles for incorporation into a liquid dosage form, a three-step process was applied that is summarized in Table 1.
TABLE 1Flow chart for the production of multi-layered particles according to theinvention, comprising (+)-3-[(N-(2-(3,4-dimethoxy-phenyl)ethyl)-piperidin-3-(S)-yl)-methyl]-(7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepin-2-on hydrochloride as active ingredient.startingstepmaterialCoatingresult1inert coredrug layering withIR pelletsparticlespharmaceutically activeingredient and HPMC / magnesium stearate2IR pelletsseal coating with PVPSC (seal coated)K 30 / Talc / colloidalsiliciumdioxidepellets3SC pelletstaste masking coating withfinal multi-EC / HPMC / magnesiumlayered par...
example 2
Preparation of a Liquid Pharmaceutical Composition
[0087]In order to prepare a liquid pharmaceutical composition, the final multi-layered particles comprising the active ingredient ciloradine prepared in the way explained above were incorporated into an oily liquid. This liquid consisted of a mixture of Medium chain triglycerides (Miglyol® 821, bought from Sasol, Hamburg, Germany), a hydrophilic colloidal silicium dioxide (Aerosil® 200, Evonik), a hydrophobic colloidal silicium dioxide (Aerosil ® R972, Evonik) and meat flavor, at the weight ratios listed in Table 7 below.
TABLE 7Liquid pharmaceutical composition comprising multi-layeredparticles comprising (+)-3-[(N-(2-(3,4-dimethoxy-phenyl)ethyl)-piperidin-3-(S)-yl)-methyl]-(7,8-dimethoxy-1,3,4,5-tetrahydro-2H-3-benzazepin-2-on hydrochloride.AmountComponent[% (w / w)]medium chain triglycerides (Miglyol ® 821)93.23hydrophilic colloidal silicium dioxide (Aerosil ® 200)4.44hydrophobic colloidal silicium dioxide (Aerosil ® R972)1.82meat fl...
example 3
Treatment of Cats Suffering from HF Due to HCM
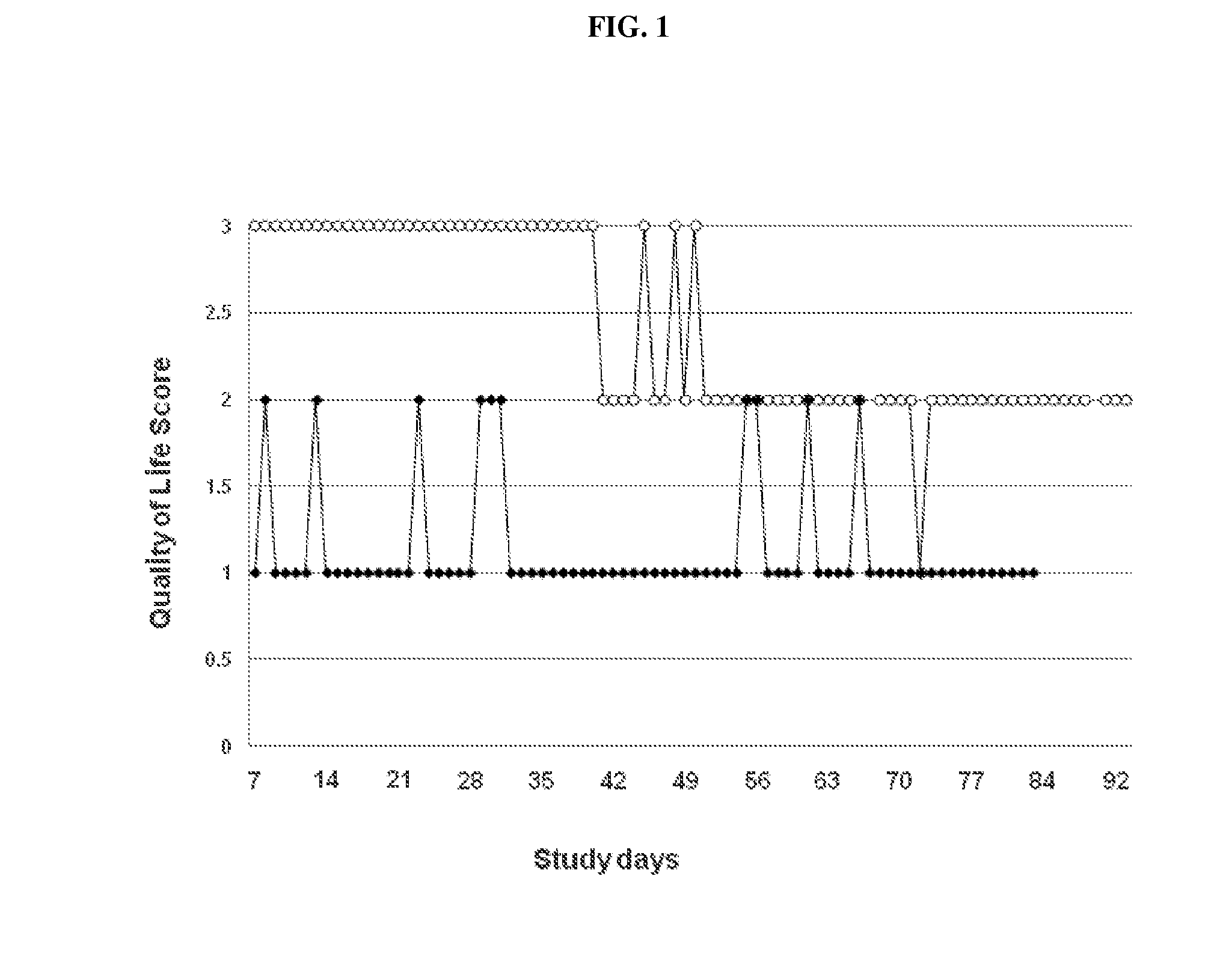
[0090]A blinded, controlled, randomised, efficacy field study was conducted with HCM cats receiving cilobradine (initial dose: 0.2 mg / kg po. twice daily) or a placebo (dose: 0.0 mg / kg po. twice daily) for 85 days. The dose of cilobradine could be adjusted during the study (dose range 0.1-0.5 mg / kg per-oral twice daily). The administration of furosemide was allowed in both groups. All cats were hospitalised in the first week of the study (study days 1-6), and they were allowed to go home afterwards (study days 7-85).
[0091]Cats with a diagnosis of HCM were included in the study. The diagnosis of HCM was established by using echocardiography showing global, regional or segmental thickness of the interventricular septum and / or left ventricular free-wall in diastole greater than 6 mm. Further inclusion criteria were: systolic blood pressure less than 170 mmHg; serum thyroxine (T4) was normal; and body weight was higher than 2 kg.
[0092]The own...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com