Fibronectin type iii domain-based multimeric scaffolds
a multimeric scaffold and fibronectin technology, applied in the field of antibody mimetics, can solve the problems of complex structure of heterotetrameric molecules, difficult to express most antibodies, and expensive mammalian cell expression systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Design of Various Multivalent Tn3 Formats
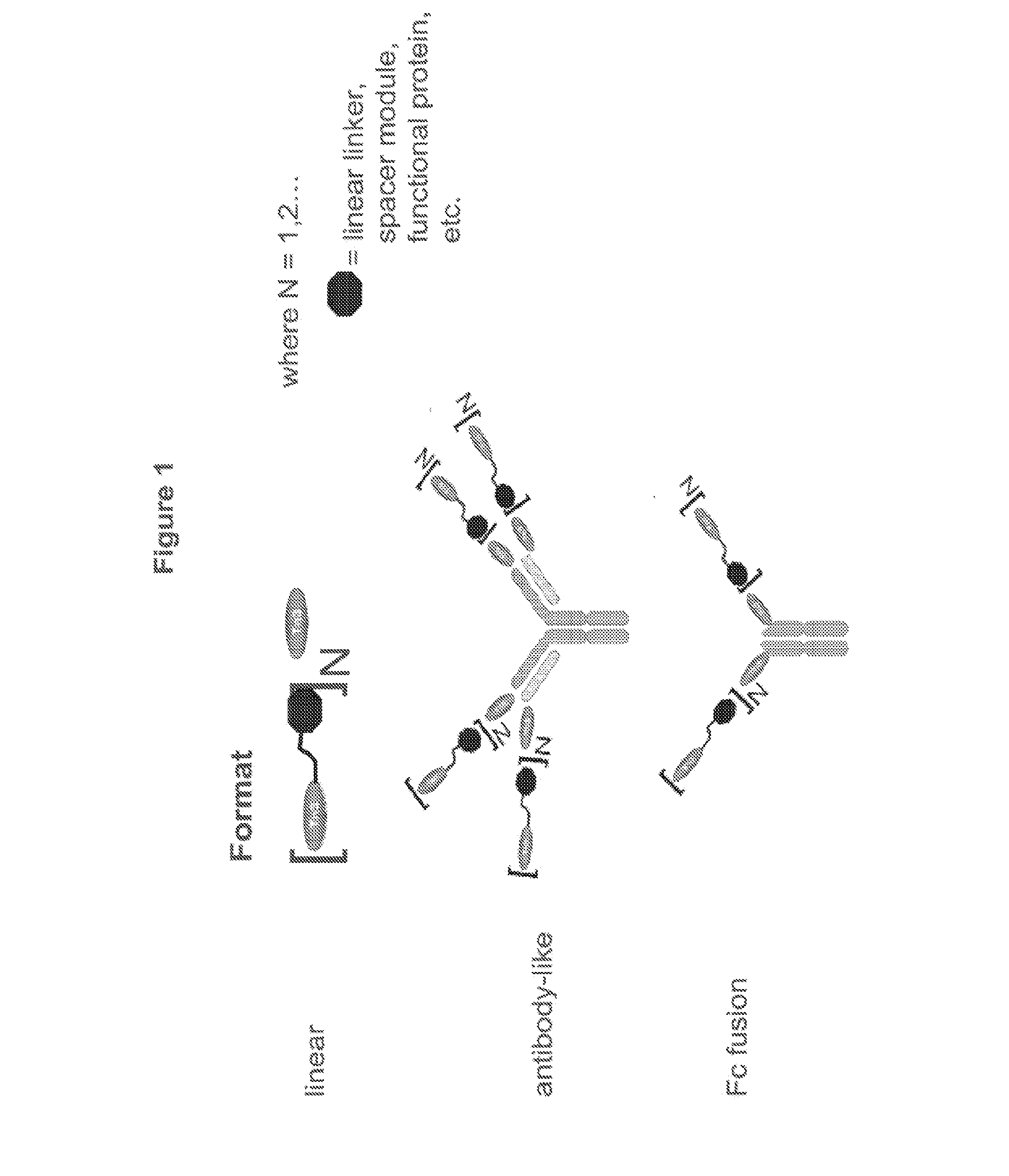
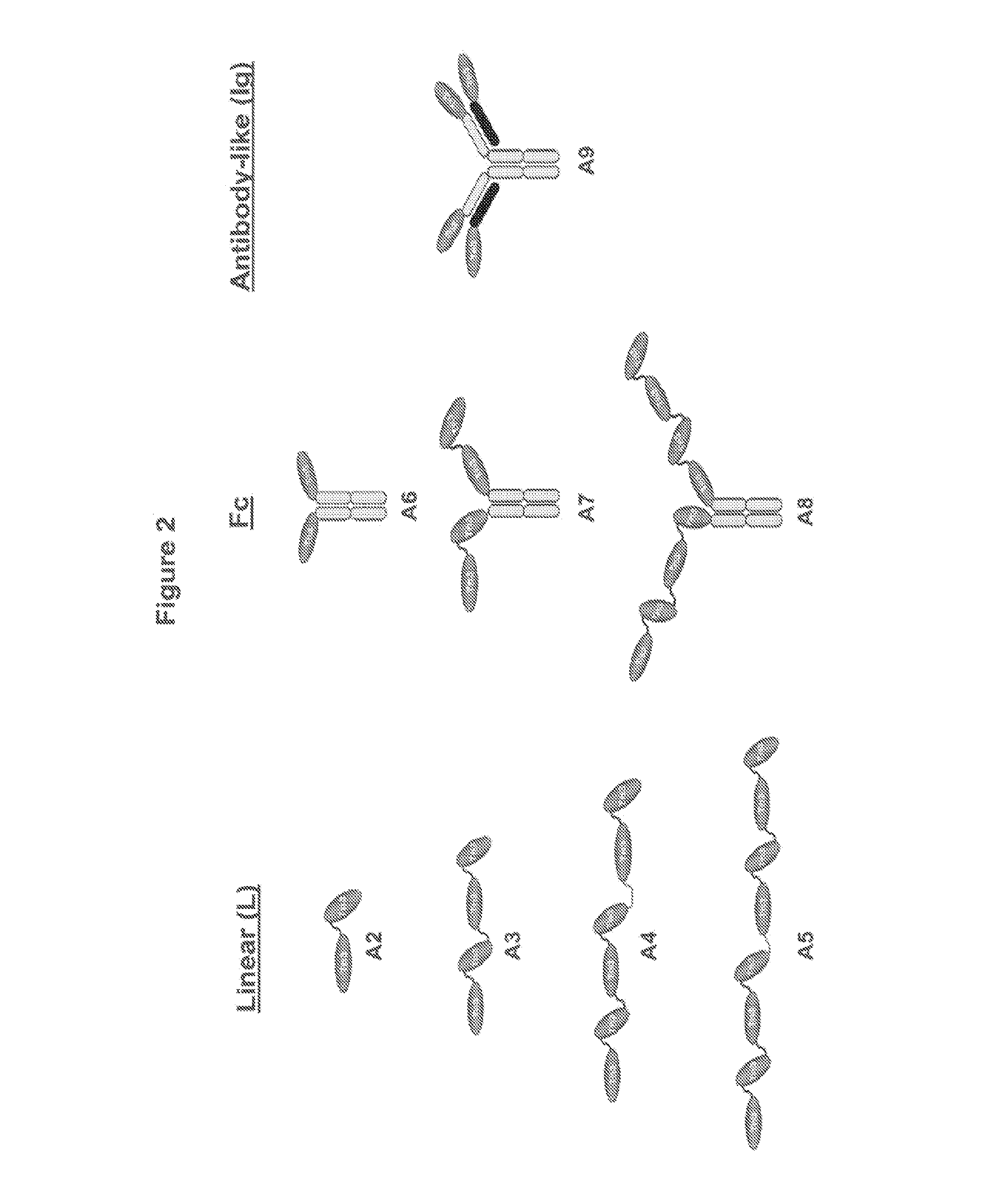
[0671]Multivalent formats of the Tn3 scaffold have been designed. The multivalent formats contain one or more Tn3 modules fused to themselves, fused to other protein motifs that can oligomerize, or fused to themselves and to other protein motifs that can oligomerize are shown in FIG. 1. In each case, the resulting molecular entity contains at least 2 Tn3 modules. The polypeptide linkers connecting the Tn3 modules to each other or to other protein motifs can be structured or unstructured and with or without a function. Three exemplary classes of multivalent Tn3 scaffold proteins are specifically provided: (i) linear (L) multivalent proteins containing Tn3 modules fused to each other via a polypeptide linker; (ii) antibody-like (Ig) multivalent proteins containing one or more linearly fused Tn3 modules fused to the light and heavy chains of an antibody or antibody fragment and (iii) Fc-containing multivalent proteins containing one or more line...
example 2
Expression and Purification of Multivalent TRAIL R2-specific Tn3-containing Proteins
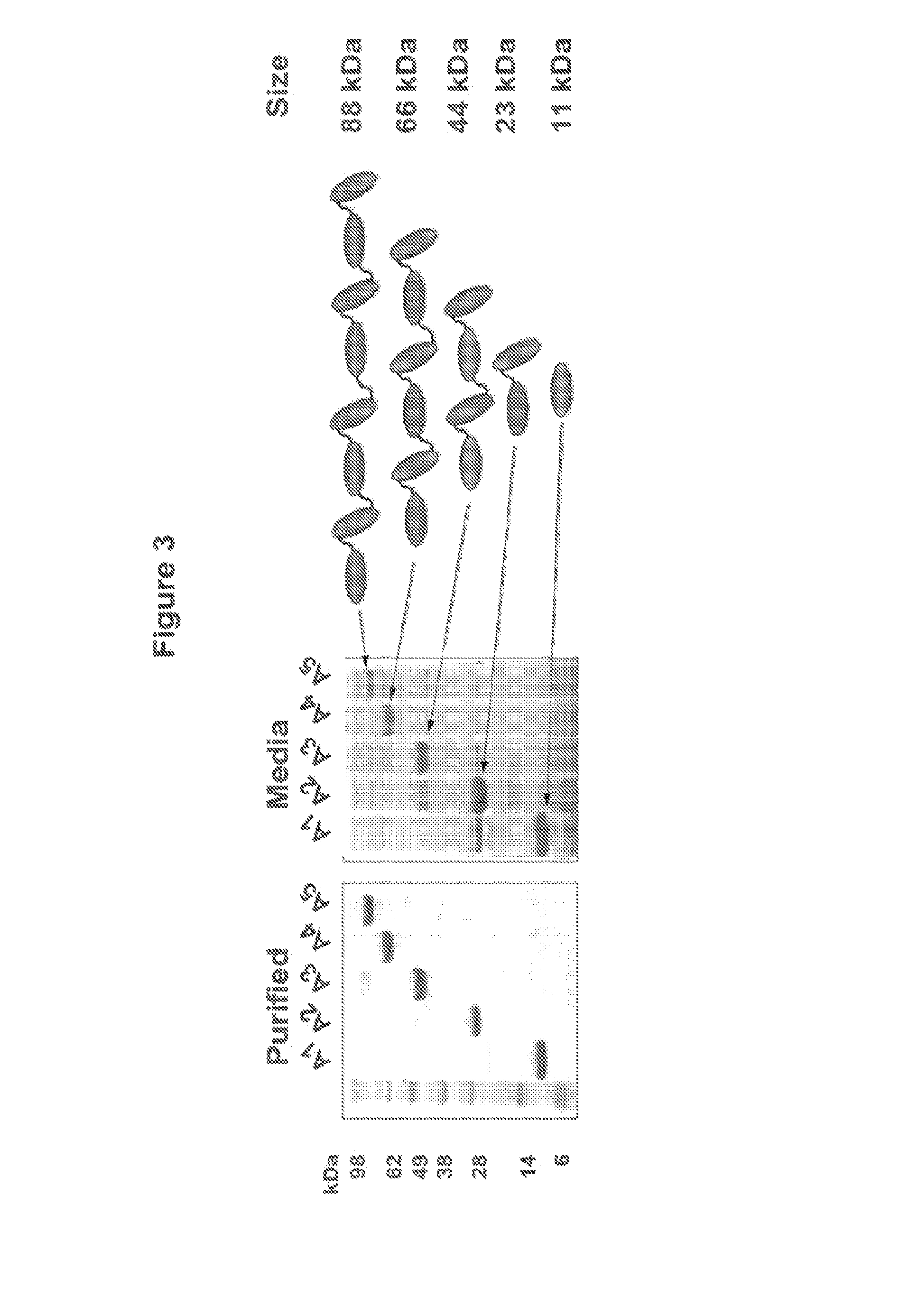
[0672]A series of eight multivalent Tn3-module containing scaffold proteins (also referred to as “Tn3 proteins” or “Tn3 scaffolds”) with binding specificity for human TRAIL R2 were prepared. Examples were prepared from each of the three multivalent formats described in Example 1, and all of these proteins presented 2 or more of the TRAIL R2-binding Tn3 module A1 (clone 1E11, G6 or 1C12). For several TRAIL R2-specific multivalent Tn3 protein, a corresponding control Tn3 protein (clone DE a Tn3 domain specific for the Synagis® antibody) that did not bind TRAIL R2 was also generated, differing only in the sequence and binding specificity of the component Tn3 modules. Tn3 clone D1 is a Tn3 protein wherein the BC, DE, and FG loops of a 1E11 clone are replaced with alternative loops with sequences corresponding to SEQ ID NO: 99, 38, and 107, respectively (see TABLE 4). Sequence identity numbers of the mult...
example 3
TRAIL R2Binding Affinity for Mono- and Polyvalent Tn3 Proteins
[0682]To measure the effect of Tn3 valency on binding affinity for a series of TRAIL R2-specific Tn3 proteins, a competition ELISA experiment was performed. A 96-well NUNC MaxiSorp plate (Thermo Fisher, Rochester, N.Y.), was coated with A9(1C12) (SEQ ID NO: 154+SEQ ID NO: 145) a TRAIL R2 specific scaffold in an antibody-like format, in PBS at 2 μg / ml overnight at 4° C. Plates were blocked with PBS 0.1% Tween 20+10 mg / ml BSA. Dilutions of A1 (1E11 monomer), and linear format A2 (1E11 bivalent) or A3 (1E11 tetravalent) multimeric scaffolds were incubated on the coated plate with 0.75 nM of biotinylated TRAIL R2-Fc for two hours at room temperature in PBS 0.1% Tween 20+1 mg / ml BSA, washed. Bound biotinylated TRAIL R2Fc was detected with streptavidin HRP, TMB, and neutralized with acid. Absorbance was read at 450 nm. Data is shown in FIG. 4. Binding affinities (IC50) are shown in TABLE 7 and were calculated as the concentrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com