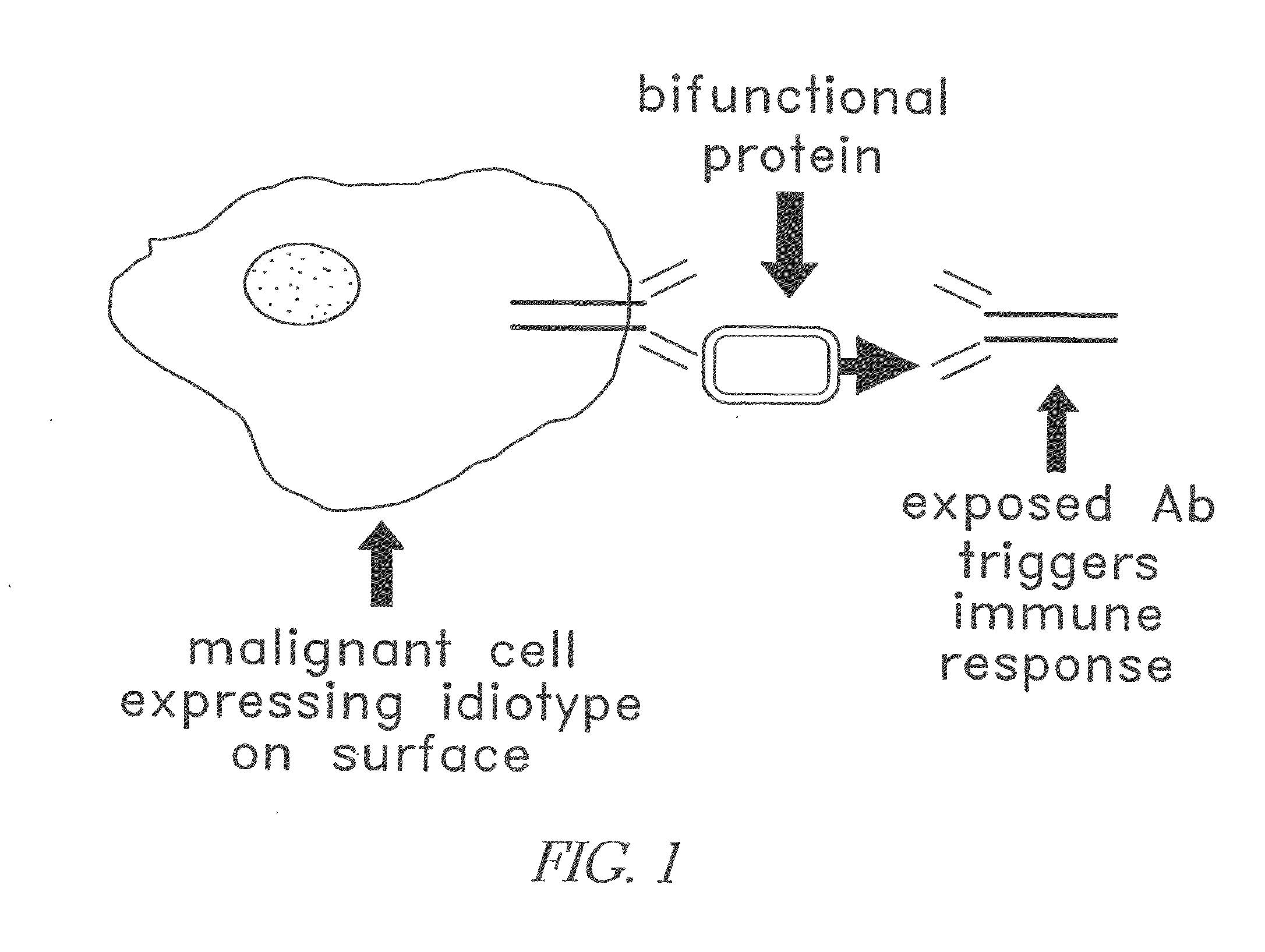
Modular targeted therapeutic agents and methods of making same
a targeted therapeutic agent and module technology, applied in the field of modules targeted therapeutic agents and methods of making same, can solve the problems of potentially significant health risks of exposure to such agents, and achieve the effects of enhancing efficacy, rapid and efficient identification, isolation and production, and low cost methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of the SATA
[0197]One skilled in the art will understand that the SATA can be produced in a number of different ways. The protocols described below in the following examples can be used for SATAs that have both a puromycin and a crosslinker on the tRNA, or that have a puromycin on the tRNA and a crosslinker on the mRNA. Where the crosslinker is on the mRNA, Example 4, below, provides guidance. The following protocol is also instructive for Linking tRNA Analogs, in the sense that Linking tRNA Analogs also, in a preferred embodiments, have a crosslinker on the tRNA.
[0198]For example, in a preferred embodiment, three fragments (FIG. 1) were purchased from a commercial source (e.g., Dharmacon Research Inc., Boulder, Colo.). Modified bases and a fragment 3 with a pre-attached puromycin on its 3′ end and a PO4 on its 3′ end were included, all of which were available commercially. Three fragments were used to facilitate manipulation of the fragment 2 in forming the monoadduct.
[01...
example 2
Production Of Psoralenated Furan Sided Monoadducts
[0207]UV Light Exposure of RNA: DNA Hybrids
[0208]Equal volumes of 3 ng / ml RNA:cRNA hybrid segments and of 10 μg / ml HMT both comprised of 50 mM NaCl were transferred into a new 1.5 ml capped polypropylene microcentrifuge tube and incubated at 37° C. for 30 minutes in the dark. This was then transferred onto a new clean culture dish. This was positioned in a photochemical reactor (419 nm peak Southern New England Ultraviolet Co.) at a distance of about 12.5 cm so that irradiance was ˜6.5 mW / cm2 and irradiated for 60-120 minutes.
[0209]Removal of Low Molecular Weight Protoproducts
[0210]1000 of chloroform-isoamyl alcohol (24:1) was pipetted and mixed by vortex. The mixture was centrifuged for 5 minutes at 15000×g in a microcentrifuge tube. The chloroform-isoamyl alcohol layer was removed with a micropipette. The chloroform-isoamyl alcohol extraction was repeated once again. Clean RNA was precipitated out of the solution.
[0211]Alcohol Prec...
example 3
Production of the SATA Using Pseudouridine
[0275]As discussed above, one skilled in the art will appreciate that the SATA, Linking tRNA Analog and Nonsense Suppressor tRNA can be produced in a number of different ways. FIG. 5 shows the chemical structures for uridine and pseudouridine. Pseudouridine is a naturally occurring base found in tRNA that forms hydrogen bonds just as uridine does, but lacks the 5-6 double bond that is the target for psoralen. Pseudouridine, as used herein, shall include the naturally occurring base and any synthetic analogs or modifications. In a preferred embodiment, the SATA was produced using pseudouridine. Linking tRNA Analog can also be produced using pseudouridine. Specifically, in a preferred embodiment, three fragments (FIG. 1) were purchased from a commercial source (Dharmacon Research Inc., Boulder, Colo.). Modified bases and a fragment 3 (“Fragment 3”) with a pre-attached puromycin on its 3′ end and a PO4 on its 3′ end were included, all of which ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
| Interaction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com