Engineering microbes and metabolic pathways for the production of ethylene glycol
a technology of metabolic engineering and ethylene glycol, which is applied in the direction of lyases, racemaces/epimerases, transferases, etc., can solve the problems of significant waste, achieve the effect of increasing the amount of 2-phosphoglycerate, increasing the amount of 3-phosphoglycerate, and reducing the flux to 2-phosphoglycera
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
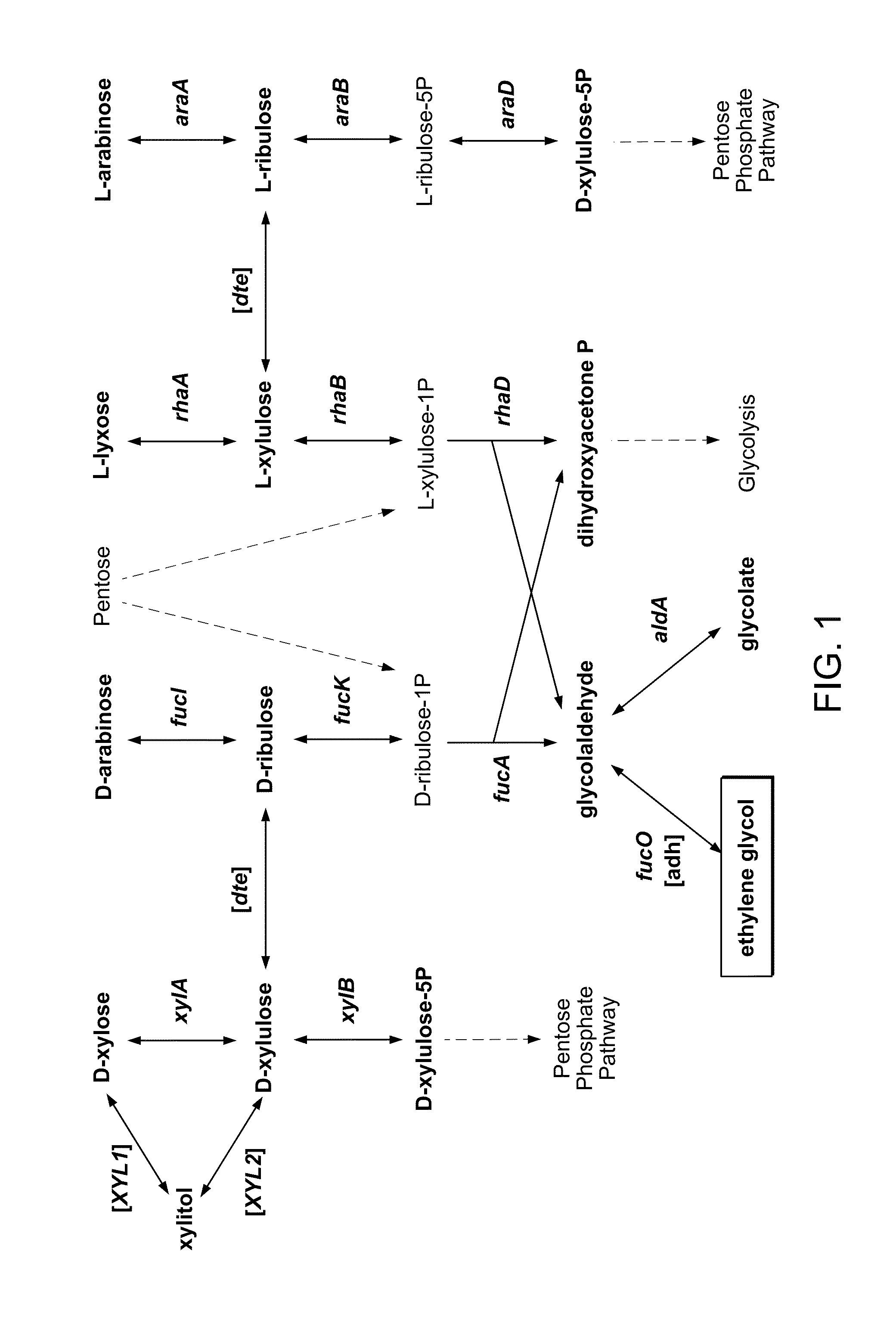
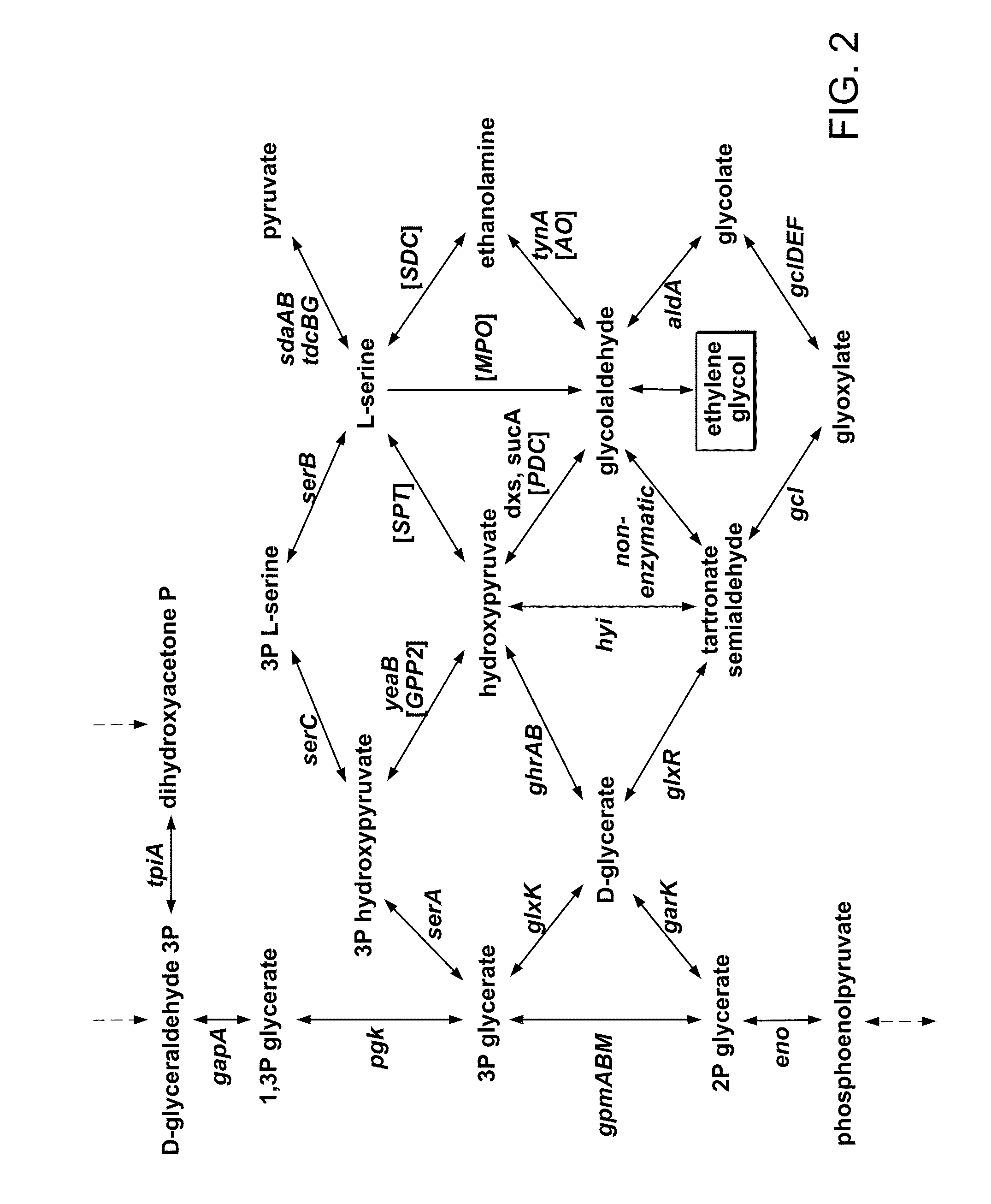
[0149]Researching E. coli for the biological production of ethylene glycol (EG) from sugars, we noted the pathway for the degradation of D-arabinose in K-12 strains. In the pathway, the pentose intermediate D-ribulose-1-phosphate is cleaved to yield dihydroxyacetone phosphate (DHAP), which enters into glycolysis, and glycolaldehyde. Glycolaldehyde is typically oxidized to glycolate by aldehyde dehydrogenase A (encoded by the aldA gene), but as in the analogous reaction of L-lactaldehyde to L-1,2-propanediol, glycolaldehyde can also be reduced by the oxidoreductase encoded by fucO. The fluxes through these reactions depend on the availability of oxygen, so we investigated the degradation of D-arabinose under aerobic and anaerobic conditions.
[0150]Cultures of E. coli K-12 MG1655 DE3 ΔendA ΔrecA (referred to as wild-type or WT) and E. coli K-12 MG1655 DE3 ΔendA ΔrecA ΔaldA (referred to as ΔaldA) were grown aerobically and anaerobically on minimal medium with 4 g / L D-arabinose as the ca...
example 2
[0151]Though we have shown that E. coli is capable of generating ethylene glycol from D-arabinose, biomass-derived sugars, such as D-xylose and D-glucose, are much more abundant and therefore are preferred substrates. Because D-xylose is a pentose, we next pursued the utilization of D-xylose for EG production through the previously established D-arabinose degradation pathway. D-tagatose 3-epimerase (DTE) from Pseudomonas cichorii is a promiscuous enzyme that has been shown to interconvert D-xylulose and D-ribulose [Izumori 1993], intermediates of the D-xylose degradation and D-arabinose degradation pathways, respectively. Therefore, DTE (encoded by the gene here referred to as dte) can provide a path by which D-xylose can yield EG, however, conversion of D-xylulose to D-xylulose-5-phosphate, catalyzed by xylulokinase (encoded by xylB), is a competing reaction. We generated E. coli K-12 MG1655 DE3 ΔendA ΔrecA ΔaldA ΔxylB (referred to as ΔaldA ΔxylB) and transformed the strain with a ...
example 3
[0153]To improve metabolism of D-xylose through the D-arabinose degradation pathway, we attempted to overexpress the relevant enzymes: D-ribulokinase (encoded by fucK), D-ribulose-phosphate aldolase (encoded by fucA), and glycolaldehyde reductase (encoded by fucO). These enzymes were overexpressed as part of an operon in conjunction with DTE; the order of the genes in the operon was varied. All of these were grown on D-xylose, analyzed for EG production, and compared against ΔaldA cultured on D-arabinose (FIG. 8). The best strain is ΔaldA ΔxylB / p10_T5T10-dte-fucA-fucO-fucK, which outperforms the D-arabinose results. When a plasmid of a lower copy number was used (p5_T5T10-dte-fucA-fucO-fucK), the results were similar (data not shown).
[0154]To maximize EG production, we next grew our best strain, ΔaldA ΔxylB / p5_T5T10-dte-fucA-fucO-fucK, in a bioreactor. The medium of the initial batch was minimal medium with 30 g / L D-xylose; when nearly all D-xylose was consumed, additional D-xylose ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com