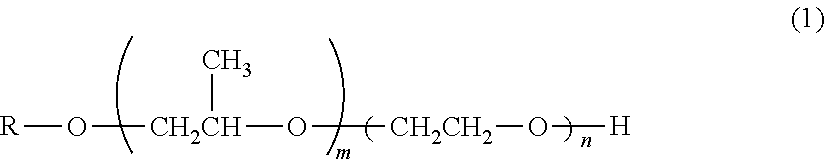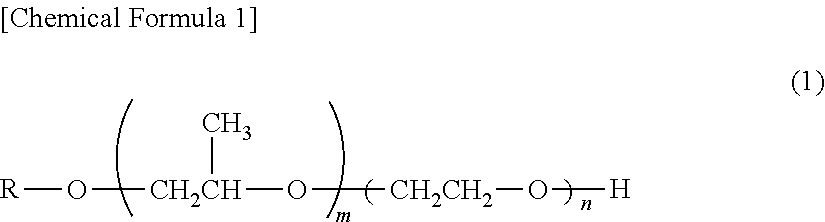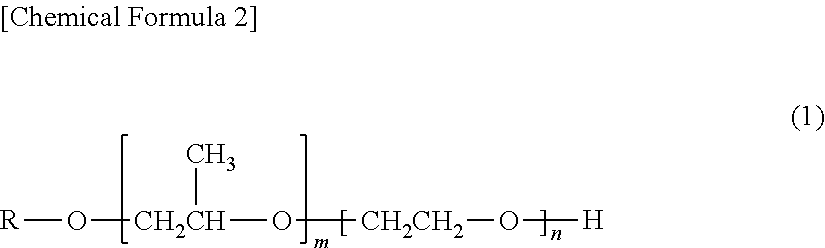Electro copper plating additive and electro copper plating bath
a technology additive, applied in the field of electro copper plating additive and electro copper plating bath, can solve the problems of poor plating, lack of throwing power, and insufficient gloss, and achieve good gloss and stable plating film
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Block Polymer
[0036]In an airtight reaction vessel, 600 g (10 mol) of n-propanol and 5.6 g (0.1 mol) of potassium hydroxide were taken, and under a nitrogen gas atmosphere, subjected to an addition reaction with 870 g (15 mol) of propylene oxide at 90 to 130 degrees C. under a pressure of 2 to 5 kg / cm2. After completing the addition reaction with the propylene oxide, the reaction vessel was cooled, and then 660 g (15 mol) of ethylene oxide was added thereto and reacted under the same conditions.
[0037]Next, 50 g of a synthetic adsorbent (Kyoward 600, manufactured by Kyowa Chemical Industry Co., Ltd.) was added to the reaction vessel and agitated at 70 degrees C. for 30 minutes, and then filtered, whereby 2070 g of polyoxyethylene (15) polyoxypropylene (15) propyl ether was obtained.
[0038](Plating Treatment and Plating Film Evaluation)
Copper sulfate (Cu2SO4•5H2O)200g / LConcentrated Sulfuric Acid50g / LNaCl115mg / L
[0039]To a copper sulfate plating solution comprising the above ...
example 2
Synthesis of B lock Polymer
[0042]In an airtight reaction vessel, 580 g (10 mol) of allyl alcohol (2-propen-1-ol) and 5.6 g (0.1 mol) of potassium hydroxide were taken, and under a nitrogen gas atmosphere, subjected to an addition reaction with 870 g (15 mol) of propylene oxide at 90 to 130 degrees C. under a pressure of 2 to 5 kg / cm2. After completing the addition reaction with the propylene oxide, the reaction vessel was cooled, and then 660 g (15 mol) of ethylene oxide was added thereto and reacted under the same conditions.
[0043]Next, 50 g of a synthetic adsorbent (Kyoward 600, manufactured by Kyowa Chemical Industry Co., Ltd.) was added to the reaction vessel and agitated at 70 degrees C. for 30 minutes, and then filtered, whereby 2050 g of polyoxyethylene (15) polyoxypropylene (15) allyl ether was obtained.
[0044](Plating Treatment and Plating Film Evaluation)
[0045]In Example 2, plating treatment was performed using a Hull cell tester with the same manner as in Example 1, except...
example 3
Synthesis of Block Polymer
[0047]In an airtight reaction vessel, 740 g (10 mol) of n-butanol and 5.6 g (0.1 mol) of potassium hydroxide were taken, and under a nitrogen gas atmosphere, subjected to an addition reaction with 580 g (10 mol) of propylene oxide at 90 to 130 degrees C. under a pressure of 2 to 5 kg / cm2. After completing the addition reaction with the propylene oxide, the reaction vessel was cooled, and then 660 g (15 mol) of ethylene oxide was added thereto and reacted under the same conditions.
[0048]Next, 50 g of a synthetic adsorbent (Kyoward 600, manufactured by Kyowa Chemical Industry Co., Ltd.) was added to the reaction vessel and agitated at 70 degrees C. for 30 minutes, and then filtered, whereby 1920 g of polyoxyethylene (15) polyoxypropylene (10) butyl ether was obtained.
[0049](Plating Treatment and Plating Film Evaluation)
[0050]In Example 3, plating treatment was performed using a Hull cell tester with the same manner as in Example 1, except that there was used ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com