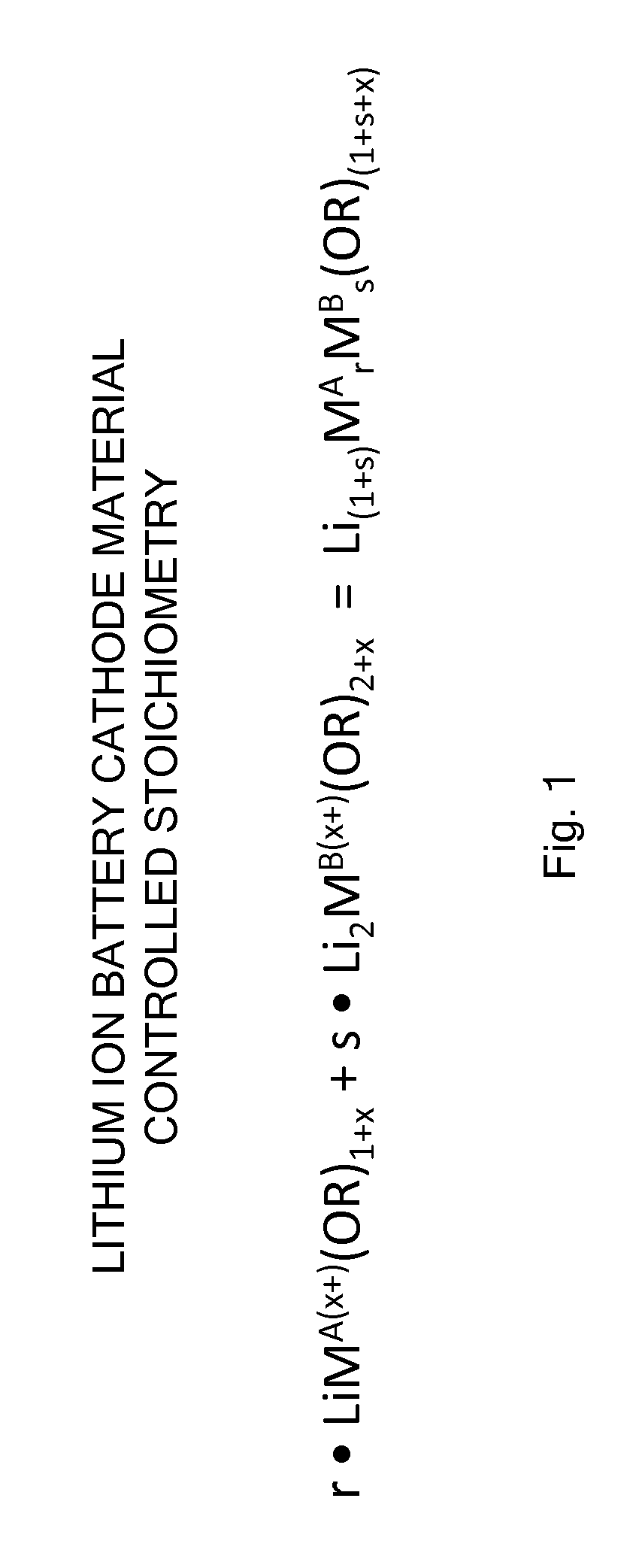
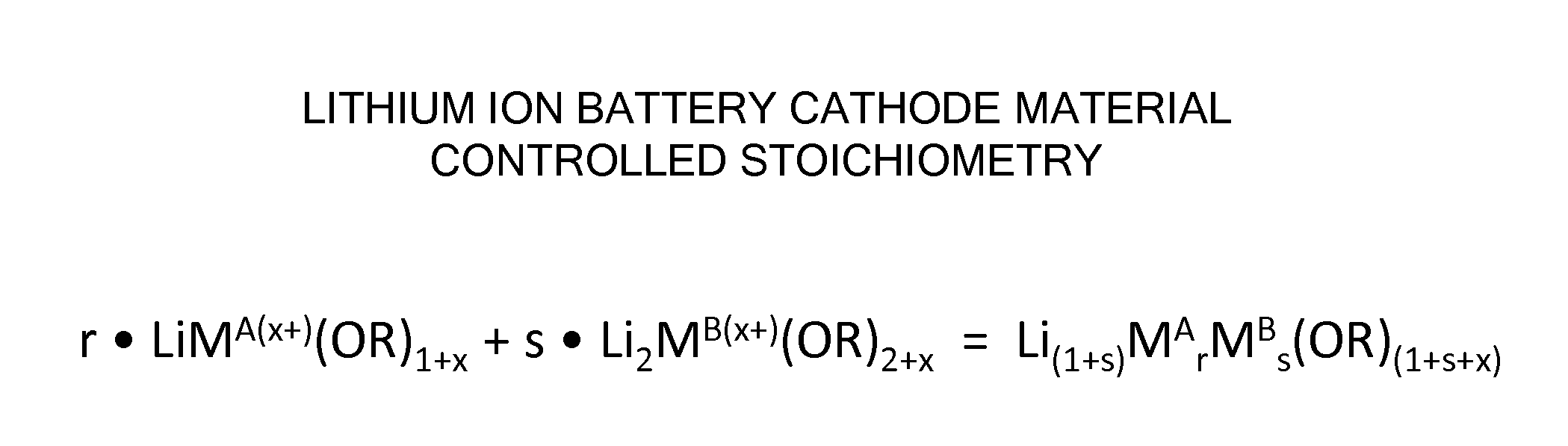Processes and compositions for multi-transition metal-containing cathode materials using molecular precursors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Compositions Containing Multiple Transition Metal Atoms
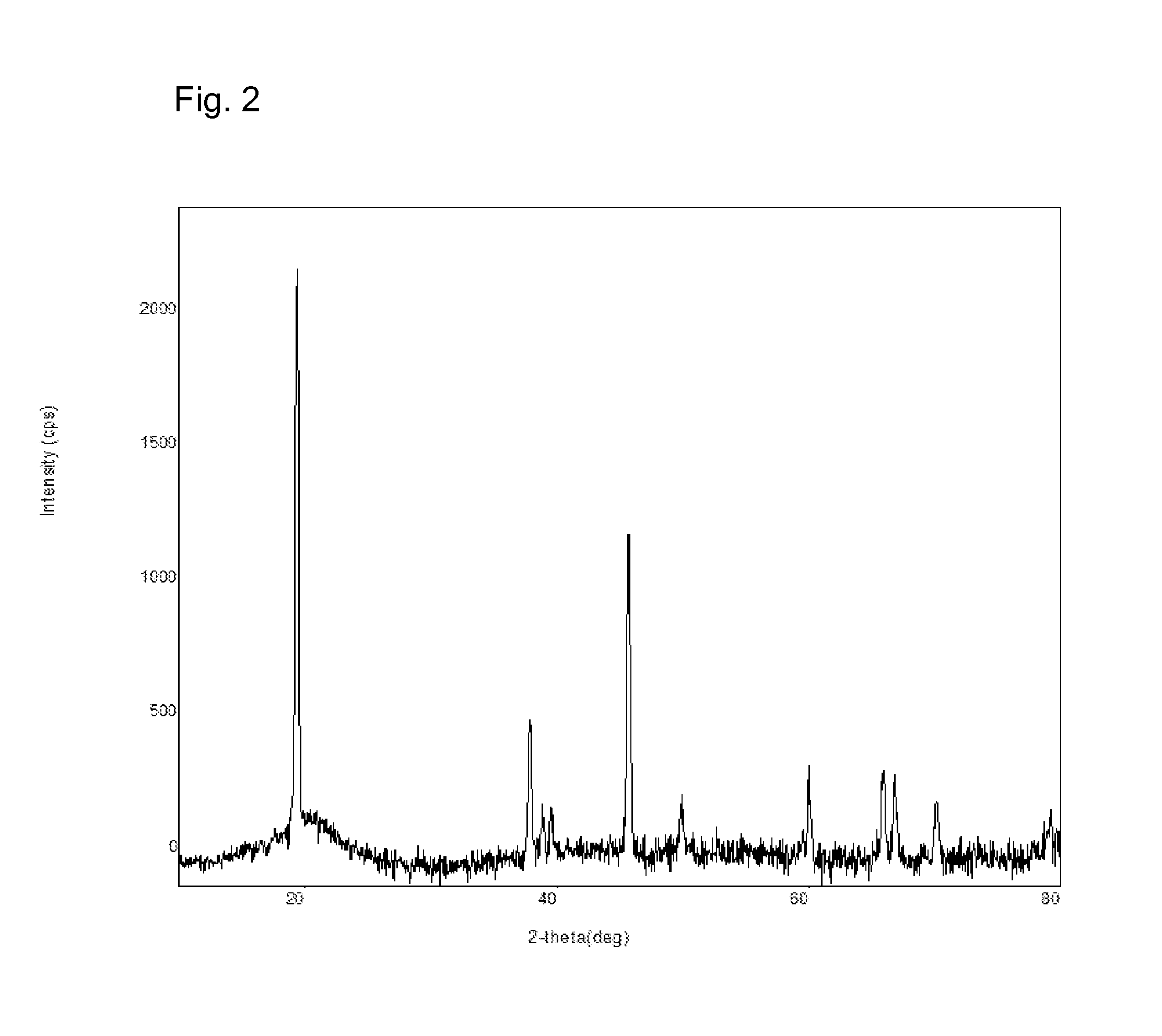
[0507]An ink containing 25 mol % LiMn(OsBu)3 and 75 mol % LiCo(OsBu)3 was prepared by mixing 0.428 g of solution “1” and 0.141 g of solution “2”. The final cathode material target for this ink was LiMn0.25Co0.75O2. The ink was spin coated onto a 50×50×0.7 mm glass substrate with a 1300 rpm spin rate and 40 s spin time under an inert atmosphere (glove box) and dried at 100° C. for 3 minutes leaving a molecular precursor film. The substrate was then heated in air at 300° C. for 5 minutes to form a LiMn0.25Co0.75O2 cathode film with a thickness of ˜120 nm.
[0508]The Li / Mn / Co ratio was found to be 0.84:0.26:0.74 by use of ICP.
[0509]Solution 1: A blue / purple solution of LiCo(OsBu)3 was prepared by dissolving 0.30 g of the molecule in 2.70 g of n-butyl acetate at room temperature (10 wt % molecule).
[0510]Solution 2: A dark brown solution of LiMn(OsBu)3 was prepared by dissolving 0.30 g of the molecule in 2.70 g of n-butyl acetate at ro...
example 2
Compositions Containing Multiple Transition Metal Atoms
[0511]An ink containing 50 mol % LiCo(OsBu)3 and 50 mol % LiMn(OsBu)3 was prepared by mixing 0.285 g of solution “1” (Example 1) and 0.281 g of solution “2” (Example 1). The final cathode material target for this ink was LiMn0.5Co0.5O2. The ink was spin coated onto a 50×50×0.7 mm glass substrate with a 1300 rpm spin rate and 40 s spin time under an inert atmosphere (glove box) and dried at 100° C. for 3 minutes leaving a molecular precursor film. The substrate was then heated in air at 300° C. for 5 minutes to form a LiMn0.5Co0.5O2 cathode film with a thickness of ˜120 nm.
[0512]The Li / Mn / Co ratio was found to be 0.79:0.50:0.50 by use of ICP.
example 3
Compositions Containing Multiple Transition Metal Atoms
[0513]An ink containing 75 mol % LiMn(OsBu)3 and 25 mol % LiCo(OsBu)3 was prepared by mixing 0.143 g of solution “1” (Example 1) and 0.422 g of solution “2” (Example 1). The final cathode material target for this ink was LiMn0.75Co0.25O2. The ink was spin coated onto a 50×50×0.7 mm glass substrate with a 1300 rpm spin rate and 40 s spin time under an inert atmosphere (glove box) and dried at 100° C. for 3 minutes leaving a molecular precursor film. The substrate was then heated in air at 300° C. for 5 minutes to form a LiMn0.75Co0.25O2 cathode film with a thickness of ˜120 nm.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap