Novel biosurfactant produced by aureobasidium pullulans
a biosurfactant and aureobasidium pullulans technology, applied in the direction of surface-active detergent compositions, organic chemistry, detergent compositions, etc., can solve the problems of water pollution, serious environmental problems, chemically synthesized surfactants are not easily degradable, etc., to confirm the activity of the surfactant and verify the novelty of the compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation of L9 from Aureobasidium Pullulans and Purification of the Same
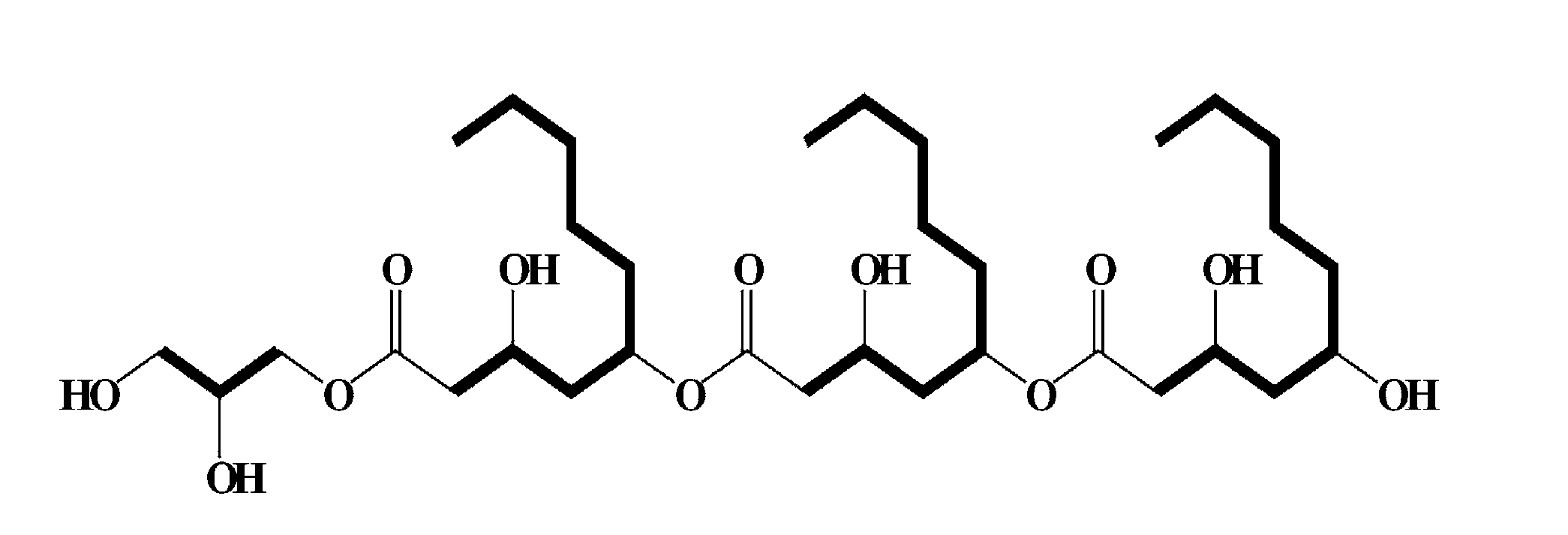
[0042]Aureobasidium pullulans L-3-GPY was deposited at the Korean Culture Center of Microorganisms (KCCM) on Jul. 5, 2011 under accession number KCCM 11200P. 100 g of a sample prepared by concentrating a broth filtrate of Aureobasidium pullulans L-3-GPY isolated from Lilium lanciforlium Thunb was dissolved in water, and flash reversed-phase column chromatography was performed. Elution was attempted using 70% and 90% aqueous methanol, and a compound having activity according to an embodiment of the present invention was eluted in 90% aqueous methanol. The eluted active ingredient was concentrated under reduced pressure to remove methanol and treated with ethyl acetate (EA) to perform phase separation. As a result of measuring activity, activity was detected in the ethyl acetate phase. Then, ethyl acetate was concentrated under reduced pressure, and silica gel chromatography was performed using a solution of chlo...
example 2
Interpretation of Chemical Structure of Active Ingredient L9 using Spectroscopy
[0043]1. Measurement and Interpretation of NMR Spectra
[0044]In order to confirm the chemical structure of active Compound L9, Compound L9 was dissolved in CD3OD, and 1H NMR, 13C NMR, DEPT, 1H-1H COSY, HMQC, HMBC, and NOESY spectra were measured and interpreted.
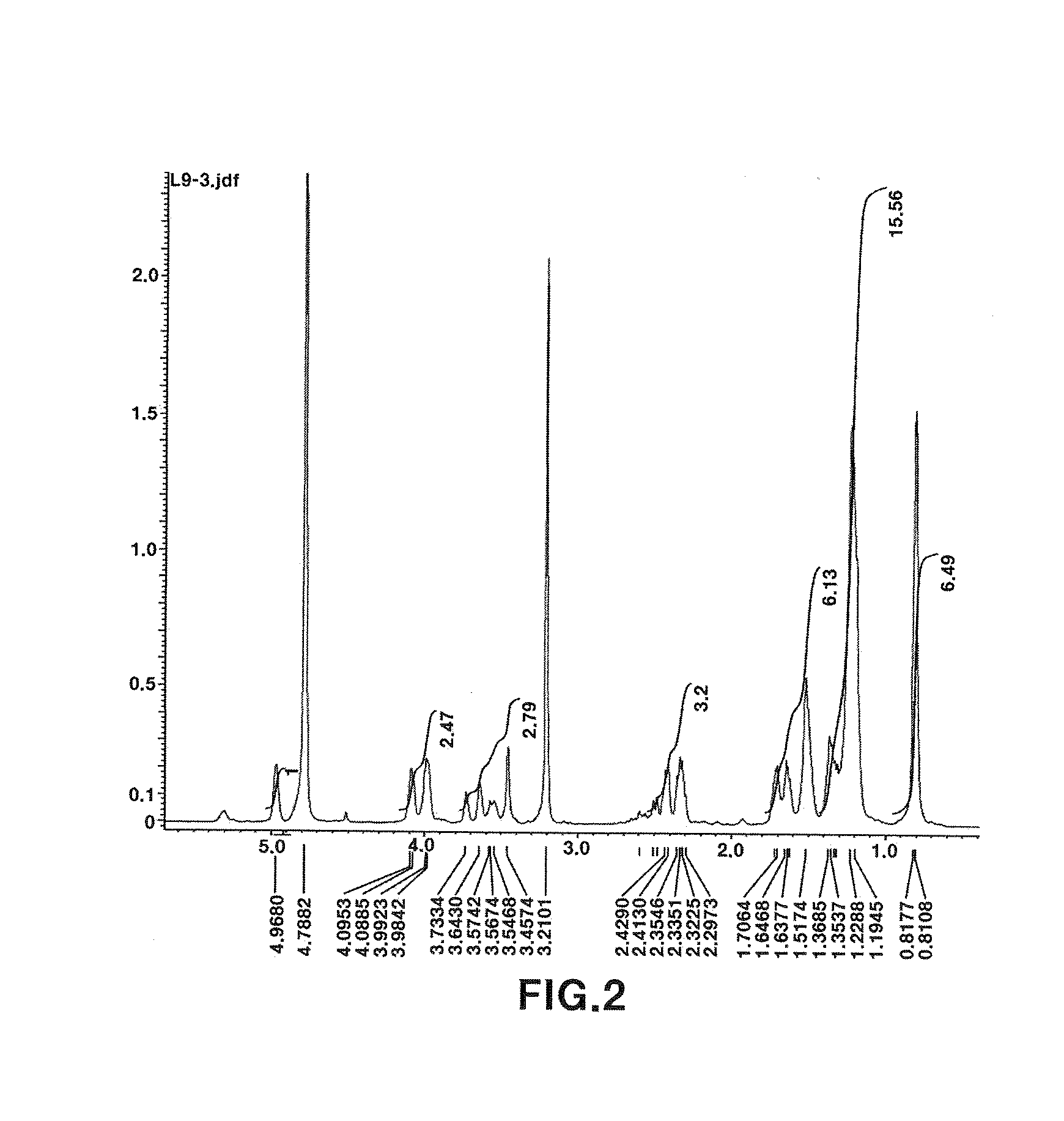
[0045]1) Measurement and interpretation of 1H NMR spectrum: As a result of measuring 1H NMR spectrum (FIG. 2), 11 protons derived from oxygenated methines (tertiary carbons) and methylenes (secondary carbons) were observed at 3.5 to 5.0 ppm. In addition, 6 protons derived from three methylenes connected to a carbonyl group were observed at 2.4 to 2.6 ppm. Further, a plurality of methylene protons derived from an alkyl group were observed at 1.2 to 1.8 ppm, and 9 protons derived from three methyl groups were observed at around 0.8 ppm.
[0046]2) Measurement and interpretation of 13C NMR spectrum: As a result of measuring 13C NMR spectrum (FIG. 3), thre...
example 3
Observation of Performance as Surfactant
[0053]1) Measurement of Surface Tension
[0054]Compound L9 isolated in Example 1 was dropped on a hydrophobic film, and surface tension variation was observed using a surface tension meter (Densitometer, Sigma 700 Densitometer (KSV Instruments Ltd., Finland)). Water was a control, and a culture medium and the size of compound L9 were compared. The results are shown in FIG. 14.
[0055]As intermolecular interaction increases, surface tension increases. Since hydrocarbons, organic polymers, and the like have week intermolecular interactions, the surface tension thereof is low.
[0056]The numerical value of the surface tension was shown as (dyne / cm (or mN / m)). When a surfactant that has a hydrophobic moiety and a hydrophilic moiety is added to water, surface tension decreases. Table 1 below shows surface tension of water, mercury, and glycerin. As the intermolecular interaction increases, the surface tension increases.
[0057]Compound L9 according to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com