Topical steroid composition and method
a technology of topical steroid and composition, which is applied in the direction of drug compositions, dermatological disorders, oil/fat/waxes non-active ingredients, etc., can solve the problems of inferior therapeutic performance of lotion-based preparations of corticosteroids compared to corresponding cream-based preparations, and achieve the effects of improving skin surface hydration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0066]In one specific procedure, a lotion is prepared in accord with the present invention utilizing the formulation of Table 2. Listed in Table 2 is a specific composition based upon the ranges set forth hereinabove.
TABLE 2Component% W / WQuantityDiisopropyl Adipate3.50%105.0gOctyldodecanol, NF10.00% 300.0gCeteth-201.00%30.0gPoloxamer 407, NF1.00%30.0gCetyl Alcohol, NF2.00%60.0gStearyl Alcohol, NF0.66%19.8gPropylparaben, NF0.10%3.0gButylparaben, NF0.05%1.5gGlycerin, USP2.50%75.0gPropylene Glycol, USP10.00% 300.0gCarbomer Homopolymer, NF0.15%4.5gSodium Hydroxide, NF0.012% 0.36gHalobetasol Propionate, USP0.05%1.5gPurified Water, USP68.978% 2069.34gTotal % 100%Theoretical Total Weight3000.0g
[0067]In this procedure, an aqueous phase is prepared by mixing water with the carbomer material, sodium hydroxide, glycerin, and propylene glycol. These materials are then mixed at a temperature of approximately 65° C. A second mixture comprising a DIA, octyldodecanol, ceteth-20, poloxamer 407, cety...
example 2
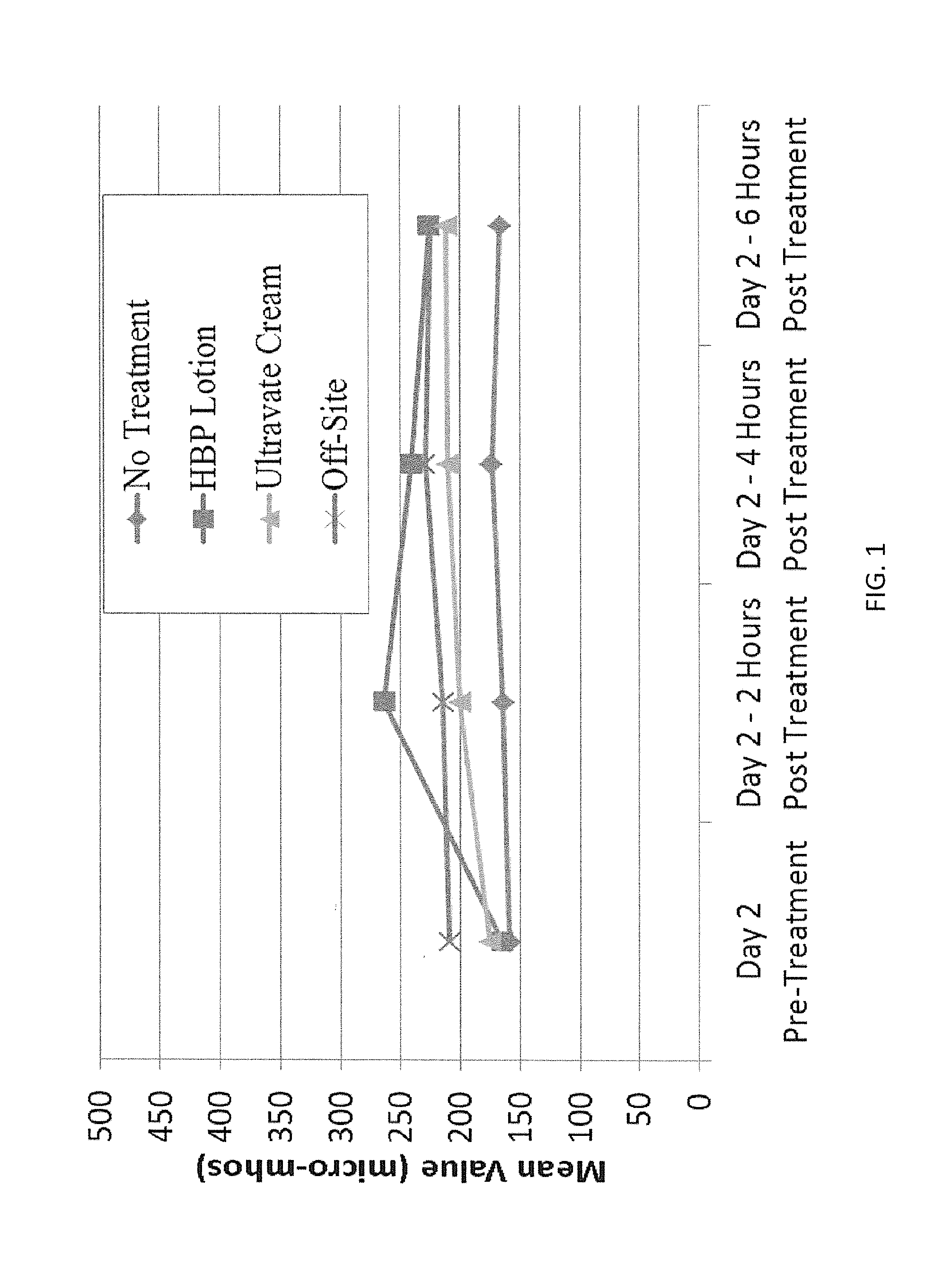
[0068]A series of studies was carried out to evaluate the properties and advantages of the compositions of the present invention. These studies were carried out utilizing an emulsified lotion preparation having a formulation in accord with Table 2 as prepared by the procedure set forth above. In a first study, the efficacy of the composition with regard to skin hydration was determined utilizing a Skicon-200 apparatus which determined the net change in skin conductance as a function of time, following application of a material. Compositions of the present invention were compared with the industry standard Ultravate® Cream and with a shaved skin control sample. The resultant data are summarized in FIG. 1. As will be seen from FIG. 1, skin conduction increased very rapidly at the 2 hour point for the composition of the present invention as compared to the Ultravate® Cream and the control. At the 4 hour point, it will be seen that skin conductance of the Ultravate® Cream has risen whil...
example 3
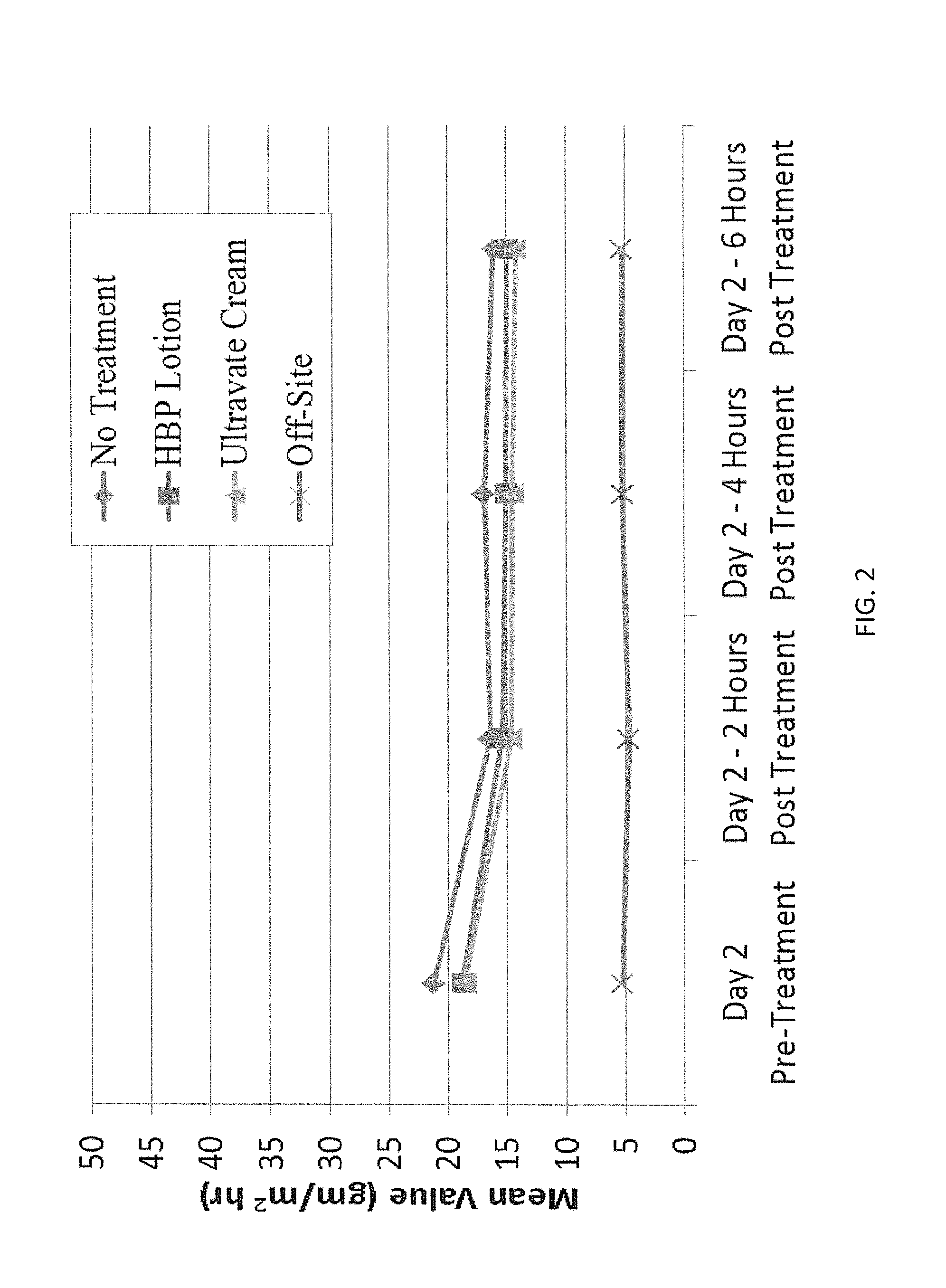
[0070]A study was carried out measuring transepidermal water loss (TEWL) of skin treated with the lotion of the present invention and skin treated with Ultravate® Cream, as compared to a dry shaved control. Computerized evaporimetry was carried out utilizing a state of the art Derm RG-1 Evaporometer; a research grade and open-chamber device based on the time proven vapor pressure gradient estimation method pioneered by Gert Nilsson. Data from this evaluation are summarized in FIG. 2 and it will be seen that over the 6 hour course of the study, the lotion of the present invention was at least as effective as the Ultravate® Cream of the prior art in preventing water loss. This finding is unexpected in view of conventional wisdom in the prior art which holds that with regard to minimizing TEWL the efficacy of ointments is greater than that of creams / gels, which is greater than that of lotions, which is greater than that of simple solutions. Therefore, the study further evidences the un...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| mean particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com