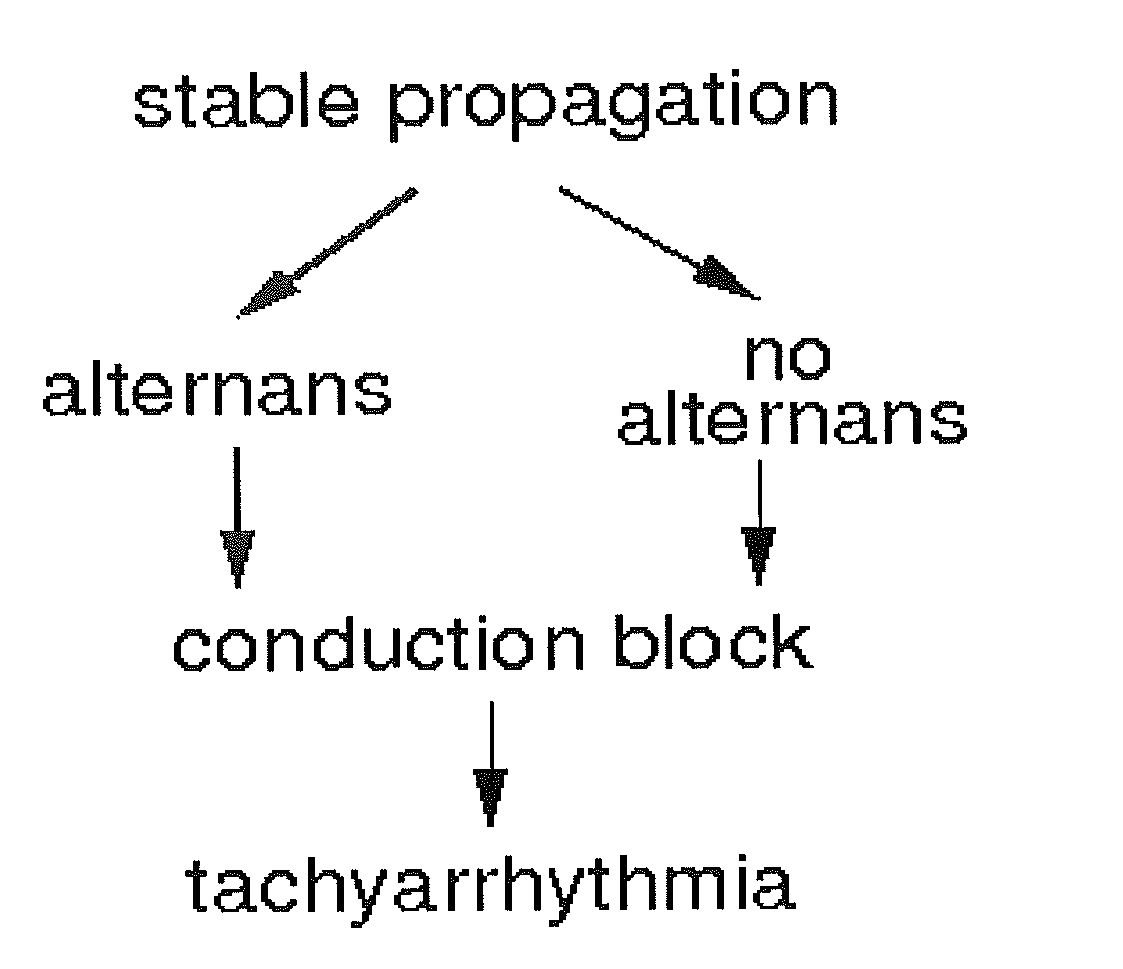
Method and system for evaluating stability of cardiac propagation reserve
a technology of propagation reserve and stability, applied in the field of methods, systems and apparatus for detecting the risk of ventricular arrhythmia, can solve the problems of unclear stratification strategy, no reliable clinical test that predicts, ep testing is an invasive procedure, and carries a non-negligible death risk, so as to prevent the accurate assessment of instabilities, increase heart rate, and reduce the effect of index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Reaction-Diffusion Model
[0101]Model Formulation.
[0102]We implement a modified Chernyak-Starobin-Cohen (CSC) reaction-diffusion model to fit experimental QT and DI intervals. The state variables of the CSC model are membrane potential u(x,t) and recovery variable v(x,t). Both u and v are dimensionless and take values between 0 and 1. The governing equations are:
∂u∂t=∂2u∂x2-(u,v)+P(x,t)and∂v∂t=ɛ(ζu+vr-v),(1)
where membrane current i(u,v) is given by:
i(u,v)=λu for u<v and (u−1) for u≧v. (2)
In (1-2), ε, λ and vr are model parameters and P(x,t) specifies the pacing pulses. At rest, u and v are equal to 0 and vr, respectively. The potential a quickly increases to 1 during the upstroke, stays near 1 during the action potential (AP), and afterwards returns to zero. The recovery variable v moves slowly toward 1 during AP and starts decreasing after AP ends. For a solitary pulse, v eventually returns to vr.
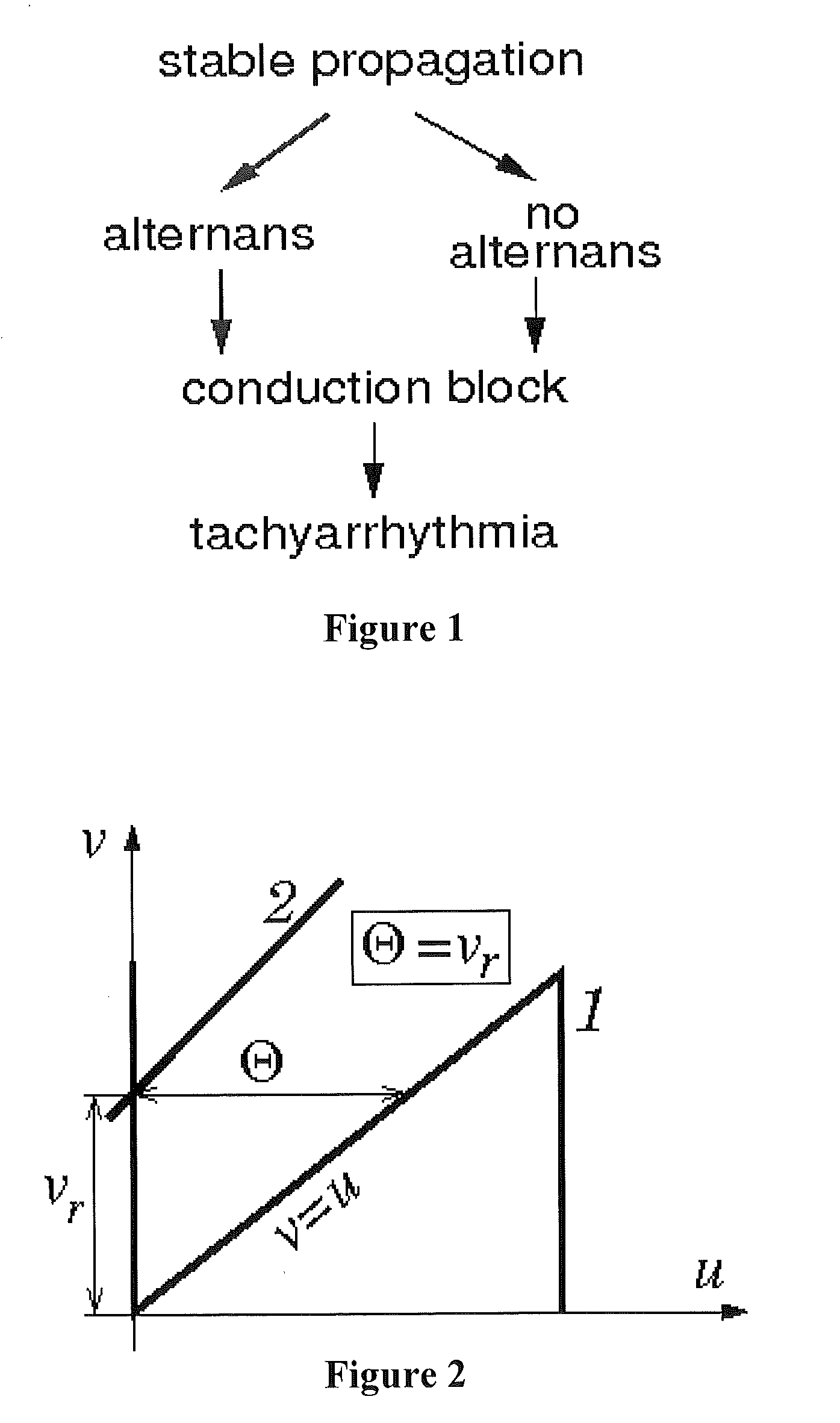
[0103]Parameter vr and the Critical Excitation Threshold.
[0104]Parameter vr has doub...
example 2
Data Collection, Processing and Construction of Restitution Portraits
[0107]The digitized original QT and RR interval signals can be recorded either during Cornell-type gradual exercise protocols or during electrophysiological (EP) studies with gradual electrical pacing. After resampling, these sequences can be used to determine the RPs. Custom software removes electrical noise and filters fluctuations and trends from the digitized signals using low-pass and high-pass frequency thresholds. The same software package can eliminate pacing stimuli without distortion of the filtered signals by implementing Daubechies 4th order wavelet [Starobin 2007].
[0108]For data acquired from the exercise test, we implement an adaptive least-mean square (LMS) algorithm to estimate QT interval fluctuations that are physiologically related to DI [Varadarajan 2009]. Using the output of the LMS filter, we compute a cross-correlation, CC, of this signal with DI fluctuations:
CC(n)=1QTlmsDI∑j=n-pn+pQTlms(j)DI...
example 3
Customizing Parameters of the CSC Model to RPs of Individual Patients
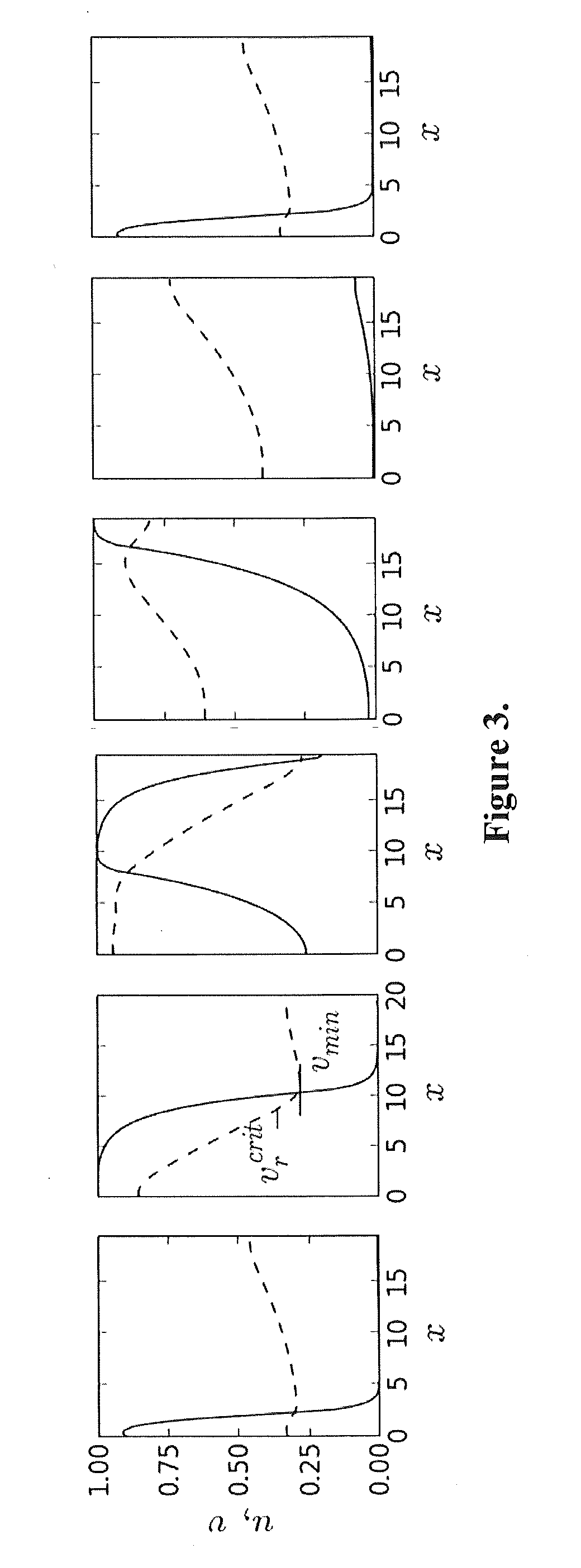
[0110]To produce results comparable to experiments, the 1D cable with the CSC model was stimulated at the left end with pacing period T, and APD and DI values were measured in the middle of the cable (FIG. 3). For all optimization runs, dimensionless APD, DI, and T values obtained from the model were converted to units directly comparable to the experimental QT, TQ, and RR intervals using the following scaling:
QT_=CmσNaAPD,TQ_=CmσNaDI,RR_=CmσNaT,(5)
where Cm and σNa are characteristic values of membrane capacitance and sodium membrane conductance. A bar over a symbol indicates the data predicted by the model.
[0111]In order to determine the SoPR values for each patient, we choose parameters of the CSC model so that the RP from the model matches the RP measured in the EP study. The CSC model (1-3) has six parameters: ε, ζ, τ, α, and β. Determining all six parameters simultaneously with a massive least-squares fit to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com